3273
Technical Committee 307 /
Comité technique 307
As mentioned above, Al leaches during H
2
O
2
percolation.
The deformation of the mechanical bridging by Al leaching
causes at the same time as organic matter decomposition. To
distinguish the influence of Al leaching on the mechanical
deformation from organic matter decomposition, shear
characteristics of the DWS for which the mechanical bridging
had been decomposed by leaching using distilled water adjust
pH 4.0 using HNO
3
which was almost same pH as H
2
O
2
solutions were also investigated in CASE 5 to 8. In acidic
condition, humic acid almost remains in DWS, although fulvic
acid is decomposed. In CASE 7 and 8, samples previously
submerged in HNO
3
repeatedly, and the specimens were
produced by dynamic compaction using the decomposed
sample.
3.2 Experimental results
The respective relations between the deviator stress and the
volumetric strain to axial strain at 50 kPa of confined pressure
was presented in Fig. 6. Volumetric swelling was slight in the
large axial strain range, and the maximum deviator stress
decreased concomitantly with the decrease in ignition loss. For
the cases of distilled water percolation, the influence of
percolation volume was not obtained. The larger dry density
typically showed higher shear strength. For the cases of pH 4.0
water submergence, maximum deviator stress slightly decreased
and cumulative Al release increased with the increment of
submergence. The influence of Al release on shear strength was
relatively smaller than those of organic matter decomposition.
The internal friction angle and the cohesion of DWS after
organic matter decomposition were, respectively, 38.6° and 0
kN/m
2
. The mechanical bridging and the organic matter
decomposition do not influence DWS cohesion. Assuming that
the cohesion is 0 kN/m
2
, the relation between the internal
friction angle and the decomposition rate of organic matter was
presented in Fig. 7. After the organic matter was decomposed
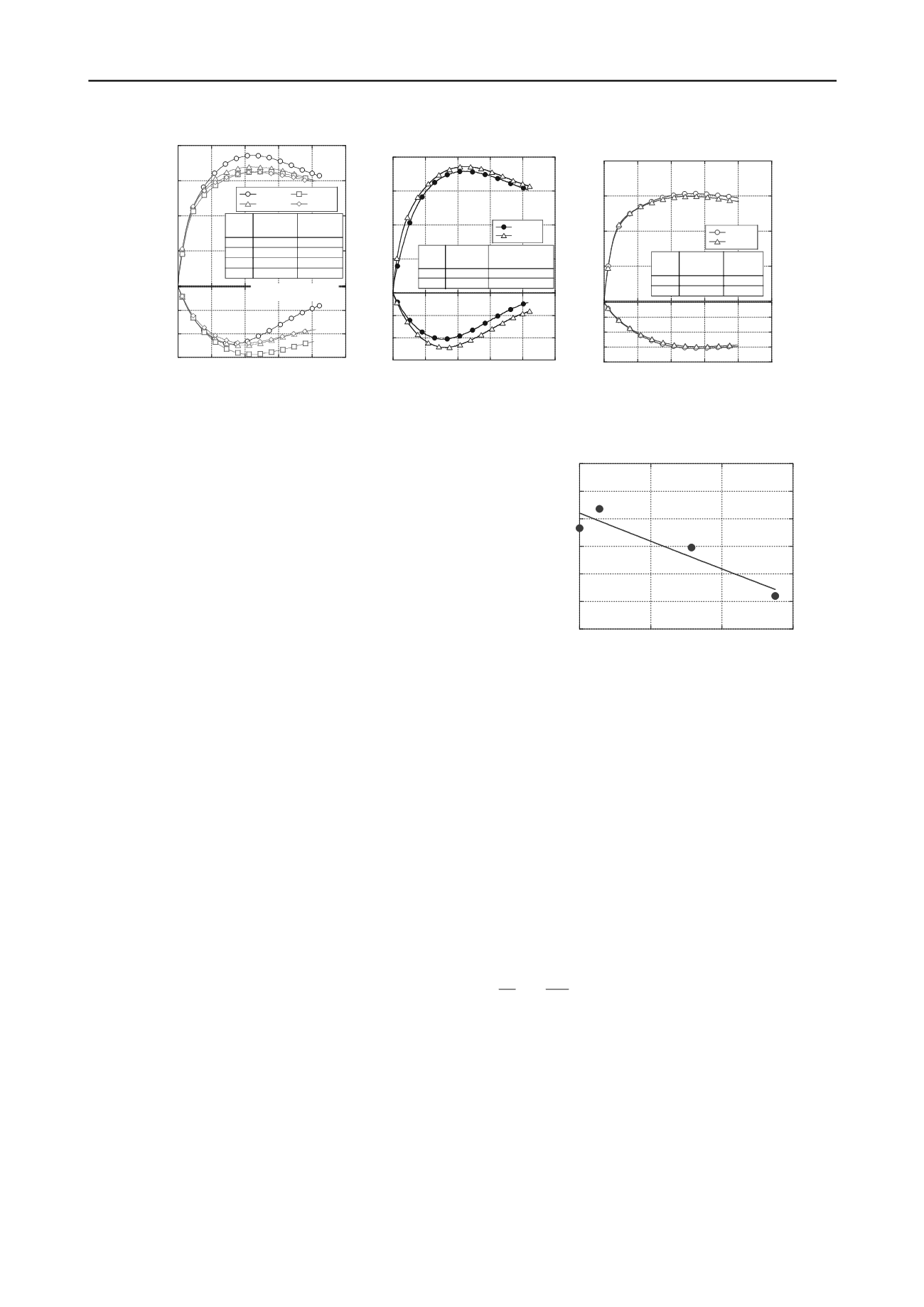
until 1.38%, the internal friction angle decreased from
approximately 38.8° to 37.6°. For the cases of pH 4.0 water
submergence, the internal friction angle decreased from 37.2° to
36.7° although the dry density was smaller than any others.
Consequently, results show that the decomposition of organic
matter remarkably affects to the DWS shear strength. Chemical
bonding between particles by the cementation of organic
compounds is possible (Mitchell and Soga, 2005). Presumably,
the strength of DWS clods decreased because the bonding was
lost through organic matter decomposition.
4 PREDICTION OF SHEAR STRENGTH TRANSITION
Al release and organic matter decomposition cause the
decrease in shear strength of DWS. To evaluate the durability of
DWS as a geo-material, this study related the change of its shear
strength the decomposition to the substantial period in
geotechnical works such as road infrastructures.
0
50
100
150
200
CASE1
CASE2
CASE3
CASE4
Deviator stress (kN/m
2
)
Ignition loss
(%)
Dry density
(Mg/m
3
)
CASE
17.51
0.821
1
17.77
0.825
2
17.12
0.815
3
16.53
0.823
4
1
2
3
0
5
10
15
20
25
Volumetric strain (%)
Axial strain (%)
CASE 2,3,4: decomposition
by hydro peroxide solution
0
50
100
150
200
CASE 5
CASE 6
Deviator stress (kN/m
2
)
Percolation volume
(mL)
Dry density
(Mg/m
3
)
CASE
500
0.817
5
3000
0.821
6
1
2
3
0
5
10
15
20
25
Volumetric strain (%)
Axial strain (%)
0
50
100
150
200
CASE 7
CASE 8
Deviator stress (kN/m
2
)
Al release
(mg/kg)
Dry density
(Mg/m
3
)
CASE
0.130
0.783
7
0.192
0.795
8
1
2
3
4
0
5
10
15
20
25
Volumetric strain (%)
Axial strain (%)
(a) H
2
O
2
percolation (b) distilled water percolation
(c) pH 4.0 water submergence
Figure 6. Relation between deviator stress and axial strain in triaxial compression tests using decomposed DWS.
37.0
37.5
38.0
38.5
39.0
39.5
40.0
0
0.5
1
Internal friction angle (deg.)
Decomposition rate of organic matter (%)
1.5
Figure 7. Relation between internal friction angle and
decomposition rate of organic matter.
4.1 Shear strength transition addressing decomposition of
mechanical bridging
Decomposition of the mechanical bridging is described as Al
leaching behavior. When DWS is used as a subgrade material
under groundwater level, Al diffusively leaches. Therefore, the
Al leaching behavior is described as a diffusion equation based
on the Fick’s law.
2
2
x
C D
t
C
e
(1)
In that equation,
D
e
is the coefficient of diffusion [m
2
/s],
C
is
the concentration [mg/L], and
x
signifies the distance from
particle surface [m]. An initial condition and boundary
conditions are shown as follows.
0
1
0
;
,0 ,
;0 ,0
;0 ,0
C C x
tC C x
t
C C x
t
where
C
0
represents the internal concentration of material
[mg/L], and
C
1
is the constant concentration [mg/L]. Assuming
C
0
is sufficiently higher than
C
1
, the cumulative release
M
is
derived.


