3040
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
O
2
and water, which were employed in this study.
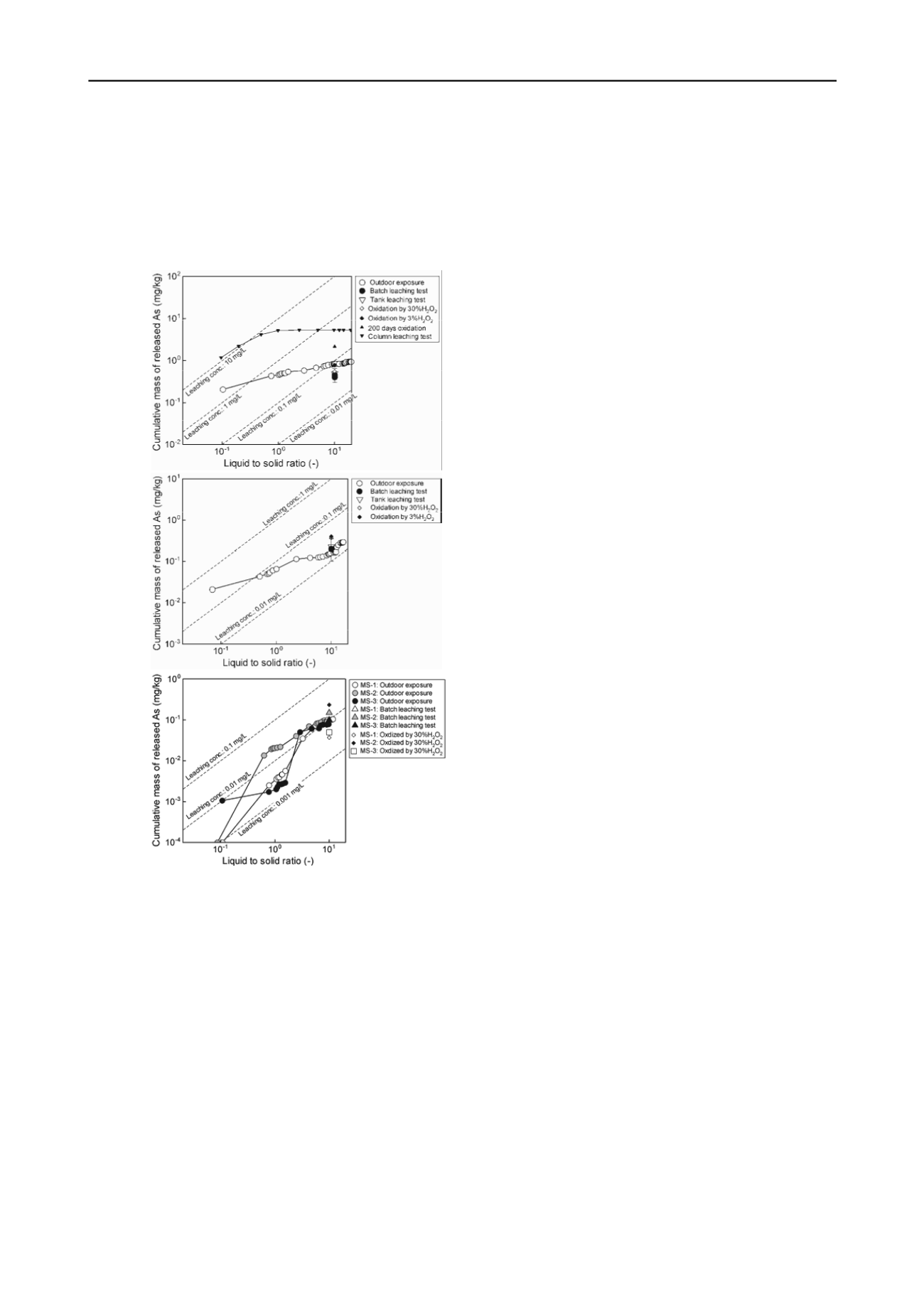
Figures 5 shows As leaching amounts of all the samples in
outdoor exposure test and several laboratory leaching tests
described in section 2.3. The leaching amounts of As from unit
weight of each rock sample are plotted with the cumulative
volume of solvent or percolated water contacting with the rock
sample during laboratory leaching tests and outdoor exposure
tests, which is represented by the liquid to solid ratio (L/S).
(a) Black shale
(b) Andesite
(c) Mudstones
Figures 5. Comparison of As leaching amounts obtained in 27 months
outdoor exposure test and laboratory leaching tests.
For the black shale (Figure 5(a)), the leaching amount at L/S
= approximately 10 reached 0.84 mg/kg, which is slightly larger
than those in the conventional batch leaching test as well as the
accelerated acidification test, which were conducted with L/S =
10. Considering that chemical activity of the black shale is
relatively since EC values of the leachate collected were largest
among all the rock samples, the chemical equilibrium achieved
in the closed batch leaching systems was likely to limit the
dissolution of As. Column leaching test gave 10 times larger
leaching amount than the outdoor exposure test, probably
because a crushed sample (< 4.75 mm in diameter) was used,
and the permeant was continuoulsy renewed in the column
leaching test. Thus, sample preparation in the laboratory
leaching test is also a key issue for the rock sample.
For the andesite (Figure 5(b)), the overall trends were almot
similar to those of the black shale. However, a slope for the
outdoor exposure test became steeper as the percolation volume
increased (L/S > 10), since aforementioned As leaching
associated with the oxidation was observed. For MS-2 and 3
(Figure 5(c)), the leaching amounts at L/S = approximately 10
were almost equal to those in the conventional batch leaching
test as well as the accelerated oxidation batch leaching test.
However, similar to the black shale, MS-1 had a high chemical
activity, and its leaching concentration in the batch leaching
tests was limited to a negligible level although 0.1 mg/kg of As
was released in the outdoor exposure test at L/S = 10.
From these testing results, the leaching amount of As
obtained in the conventional batch leaching test can be a good
index of the insitu leaching amount until L/S = 10 in the cases
of rock samples with relatively low chemical activities. The
accelerated oxidation tests using H
2
O
2
solutions can simulate
the in situ leaching amount for the safe side. However, the
chemical equilibrium may limit the leaching of trace metals in
the batch leaching test as observed in black shale and MS-1.
Pb leaching concentrations were negligible for all rock
samples. According to the aforementioned criterion for total Pb
content suggested by MLIT, all the rock samples are considered
safe in terms of Pb leaching. These testing results support the
validity of the criterion for total content of Pb.
5 CONCLUDING REMARKS
This manuscript verified several laboratory tests for estimating
the long term leaching characteristics of As and Pb in several
rock materials, by comparing the results of outdoor exposure
tests. Total contents of trace metals can be regarded possibly as
screening values to judge whether detailed evaluation of
leaching characteristics are necessary. The leaching amount of
As obtained in the conventional batch leaching test can be a
good index of field leaching amount, and the accelerated
oxidation tests can simulate the outdoor leaching amount for the
safe side. These observations confirm the validity of a series of
laboratory leaching tests as a tool to estimate the in situ leaching
behavior of heavy metals in excavated rocks.
ACKNOWLEDGEMENTS
This research was financially supported by the Grant-in-Aid for
Scientific Research (B) (No. 20360211), Japan Society for the
Promotion of Science. The authors acknowledge Ms. A. Dejima
and Ms. M. Katayama, former graduate students of Kyoto
University, for their great efforts in laboratory experiment works.
REFERENCES
Hattori, S., Ohta, T. and Kiya, H. 2003. Engineering geological study on
exudation of acid water from rock mucks -Evaluation methods of
rocks at the Hakkouda Tunnel near mine area,
Jour. Japan Soc. Eng.
Geol.
, 43 (6), 359-371.
Inui, T., Katsumi, T., Katayama, M. and Kamon, M. 2010. Effects of
friability and grain size on the leaching of heavy metals in
excavated rock materials,
Environmental Geotechnics for
Sustainable Development
, M. Datta et al. (eds.), Tata McGraw Hill,
New Delhi, 730-733.
Japanese Geotechnical Society. 2009.
Japanese Standards and
Explanations of Laboratory Tests of Geomaterials
, 314-315.
Ministry of Land, Infrastructure, Transport and Tourism, Japan. 2010.
Technical Manual on the Countermeasures against Soils and Rocks
Containing Natural-Derived Heavy Metals in Construction Works
(Draft)
, 10.
Okumura, K., Sakurai, K., Nakamura, N. and Morimoto, Y. 2007.
Environmental impacts of naturally occurring heavy metals and
countermeasures,
Journal of Geology
, 116 (6), 892-905.
Zhu, Y. and Merkel, B.J.. 2001. The dissolution and solubility of
Scorodite, FeAsO
4
·2H
2
O: Evaluation and simulation with
PHREEQC2,
Wiss. Mitt. Inst. fur Geologie
, TU Bergakedemie
Freiberg, Germany, 18, 1-12.


