3039
Technical Committee 215 /
Comité technique 215
4 DISCUSSION
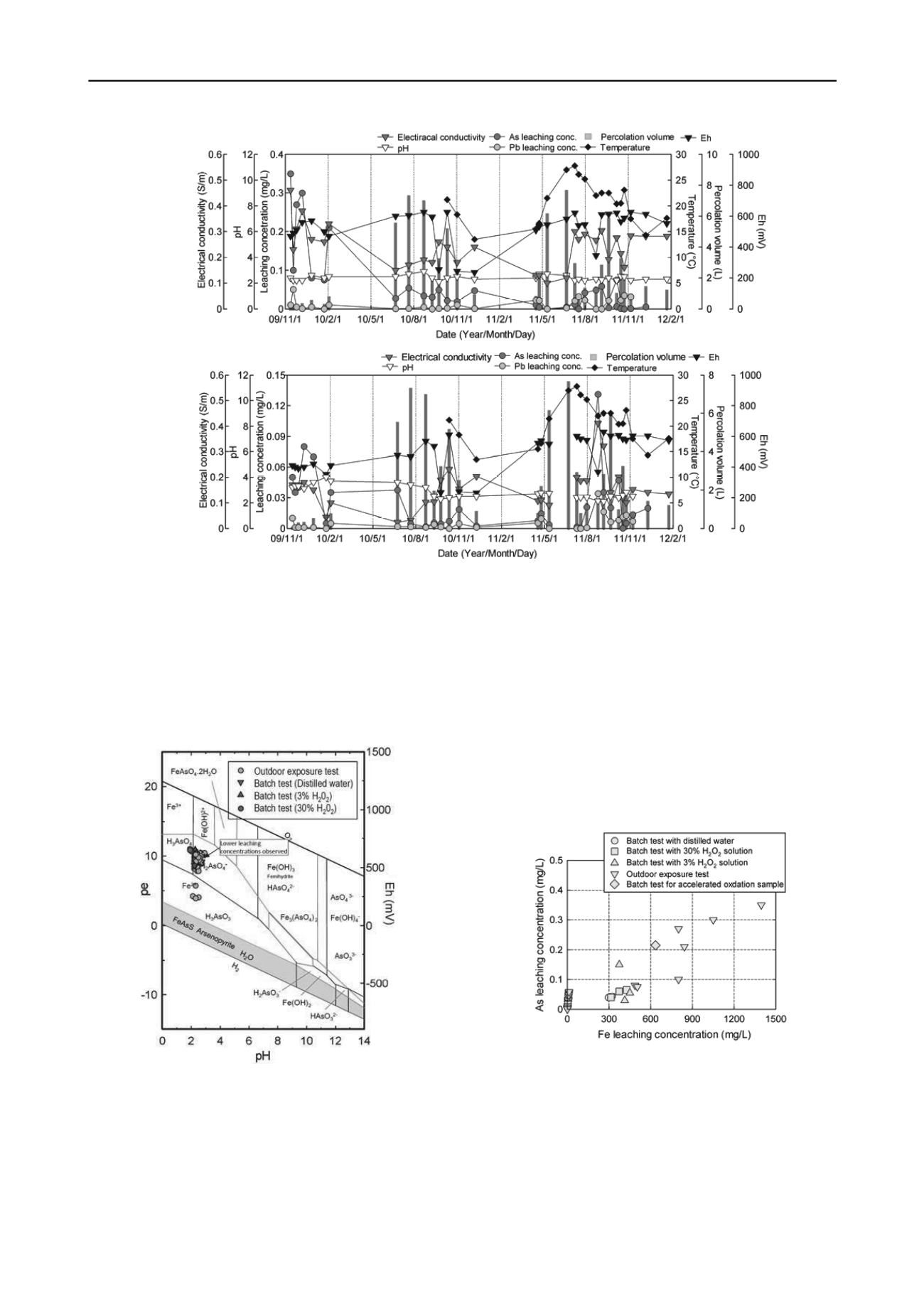
In Figure 3, pH and Eh values of the leachate samples collected
in both outdoor exposure tests and laboratory leaching tests for
black shale are plotted on the pH-Eh/pe(electron activity)
diagram of dominant forms of As and Fe in As-Fe-S-H
2
O
system (Zhu & Merkel 2001).
Figure 3. pH and Eh values observed in outdoor exposure tests and
laboratory leaching tests combined with pH-Eh diagram of dominant
forms of As and Fe in As-Fe- S-H
2
O system (Zhu & Merkel 2001).
In the outdoor exposure test, higher leaching concentrations
of As were observed even when Eh values were relatively low,
where dominant forms of iron and arsenic are Fe
2+
and H
3
AsO
3
(arsenous acid), respectively. When lower leaching
concentrations were observed (see the circle in Figure 3), the
dominant form of As is expected HAsO
4
-
, which is more easily
absorbed to iron compounds and less mobile than H
3
AsO
3
.
Comparing pH and Eh values monitored in laboratory leaching
tests with those in outdoor exposure test, pH values in the batch
test using 30% H
2
O
2
solution was lowest, and pH for 3% H
2
O
2
solution was almost similar to those in the outdoor exposure test.
This indicates that 30%H
2
O
2
solution is more influential than
outdoor exposure in more than two years in terms of
acidification, and accelerated acidification by 3% H
2
O
2
solution
is almost comparable to a few years outdoor exposure. This
trend was consistent in all the rock samples used in this study
and it can be concluded that pH changes against 3% and 30%
H
2
O
2
solutions could classify acidification potentials under the
weathered condition, but the acceleration by 30 % H
2
O
2
solution possibley overestimate the acidification progress in
outdoor even for two years.
(a) Black shale
(b) Andesite
Figures 2. Profiles of pH, Eh, heavy metal concentrations, rainfall intensity and infiltration in outdoor exposure tests
Figure 4. Relationship between Fe and As leaching concentrations in
various leaching tests
Figure 4 shows a relationship between Fe and As leaching
concentrations in both outdoor exposure tests and laboratory
leaching tests. A clear correlation between them indicates that
dissolution of iron pyrite due to oxidization is a main driver for
As leaching. In addition, Fe and As concentrations are
correlated in laboatory leaching tests as well. From these
observations, dissolution of pyrite due to oxdization was well
simulated by the accelerated acidification/oxidization methods,
such as adding H
2
O
2
solutions and long term exposure to 80%


