3031
Technical Committee 215 /
Comité technique 215
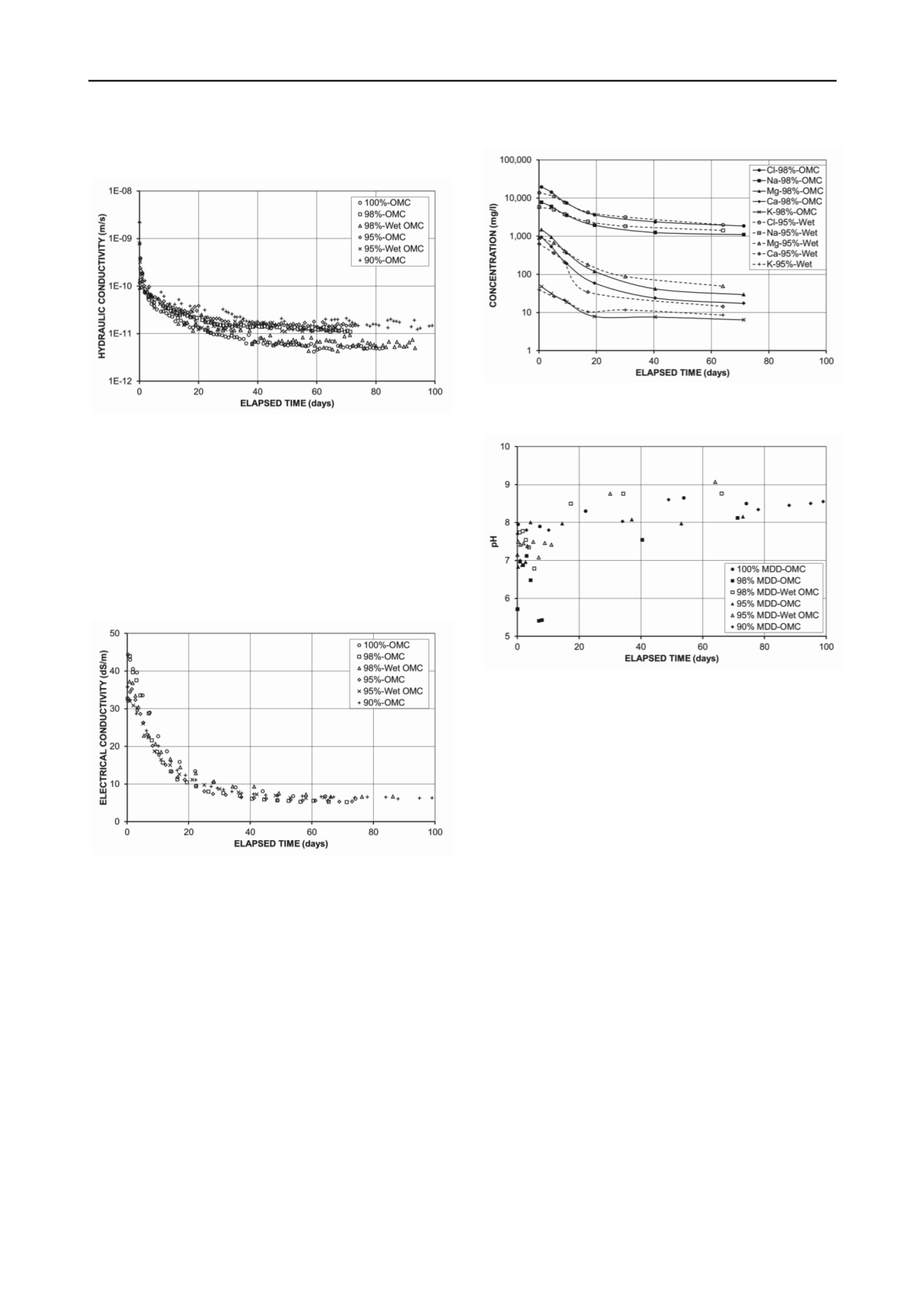
that those compacted wet of OMC tend to have lower hydraulic
conductivities than those compacted at OMC.
Figure 3. Hydraulic conductivity of SB3 specimens compacted to
various dry densities at various moisture contents.
The change in the EC of the outflow from the SB3
specimens during the hydraulic conductivity tests is shown in
Figure 4. The samples were originally saline, with an EC at
their natural moisture content much higher than that of the CW.
As the ponded CW infiltrated the compacted specimens, ionic
exchange occurred between the infiltrating CW and the original
pore water. This caused the EC of the outflow to decrease with
time and eventually approach that of the CW, as salts in the
compacted specimens were washed out, as shown in Figure 5.
Figure 4. Electrical conductivity of SB3 specimens compacted to
various dry densities at various moisture contents.
The pH of the pore water squeezed from SB3 mixed with
CW to a gravimetric moisture content of 29% was 5.7, and at
the OMC (21.8%) the pH was expected to be lower. Figure 6
shows that the pH of the outflow from SB3 specimens
compacted to various dry densities at different moisture
contents increased with time, exceeding the pH at the point of
zero charge (PZC) at the edges (E) of the kaolinite particles, in
the pH range 5 to 7 (Kretzschmar et al. 1998, Wang and Siu
2006). Below the PZC, the edges of kaolinite particles carry
positive charges, while above the PZC, the edges carry negative
charges. The faces (F) of kaolinite particles are always
negatively-charged, resulting in a lower pH than at the edges
(Wang and Siu 2006). Below the PZC, kaolinite particles tend
to develop an E-F flocculated structure. When the pH is greater
than that at the PZC, E-F interaction is prevented, since both E
and F are negatively-charged, and kaolinite particles tend to
have a dispersed structure.
Figure 5. Concentrations of major ions in outflow from SB3 specimens
compacted to 98% and 95% of MDD.
Figure 6. pH of SB3 specimens compacted to various dry densities at
various moisture contents.
The hydraulic conductivity was initially high due to a
flocculated clay structure, and decreased with time as the
kaolinite particles became aligned and developed a dispersed
structure. In addition, high clay dispersion was observed in the
upper 3 to 5 mm layer of the compacted specimens. Clay
dispersion is likely to clog the compacted pores and hence
contribute to the observed decrease in hydraulic conductivity.
Similar to the compacted specimens permeated with CW, the
hydraulic conductivity of all compacted specimens permeated
with DW also decreased with time, as shown in Figure 7.
Again, the kaolinite particles tend to develop a dispersed
structure when the PZC of the edges is exceeded. As the pH of
DW is 7, slightly above the PZC of the edges of kaolinite
particles, the infiltration of DW eventually raises the pH of the
outflow to 7, resulting in a dispersed structure and the decrease
in the hydraulic conductivity with time observed in Figure 7.
Table 3 shows that there is no a clear trend of hydraulic
conductivity of specimens moisture-conditioned and permeated
with DW and CW in the compaction mould permeameter tests.
The differences are considered to be within the accuracy of
outflow measurements at these low hydraulic conductivities,
due to susceptibility to environmental conditions such as
evaporation. Moisture-conditioning with CW and DW is likely
to affect the kaolinite structures only after mixing, or at the
beginning of the hydraulic conductivity tests. Rearrangement of
the kaolinite particles as the tests proceeded resulted in their
eventual exposure to permeating CW.
4.2
Oedometer hydraulic conductivity
Despite the reduced reliability of the oedometer test for
determining the hydraulic conductivity of a compacted clay, the
values obtained from the oedometer test data under an applied
stress of 100 kPa were reasonably comparable to, or a little


