3370
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
Figure 7 portrays the methylene blue absorption for
bentonites A and C that had undergone thermal exposure.
Results show thatthe absorbing capacities of methylene blue for
bentonitesof both kinds are reducedby thermal exposure.
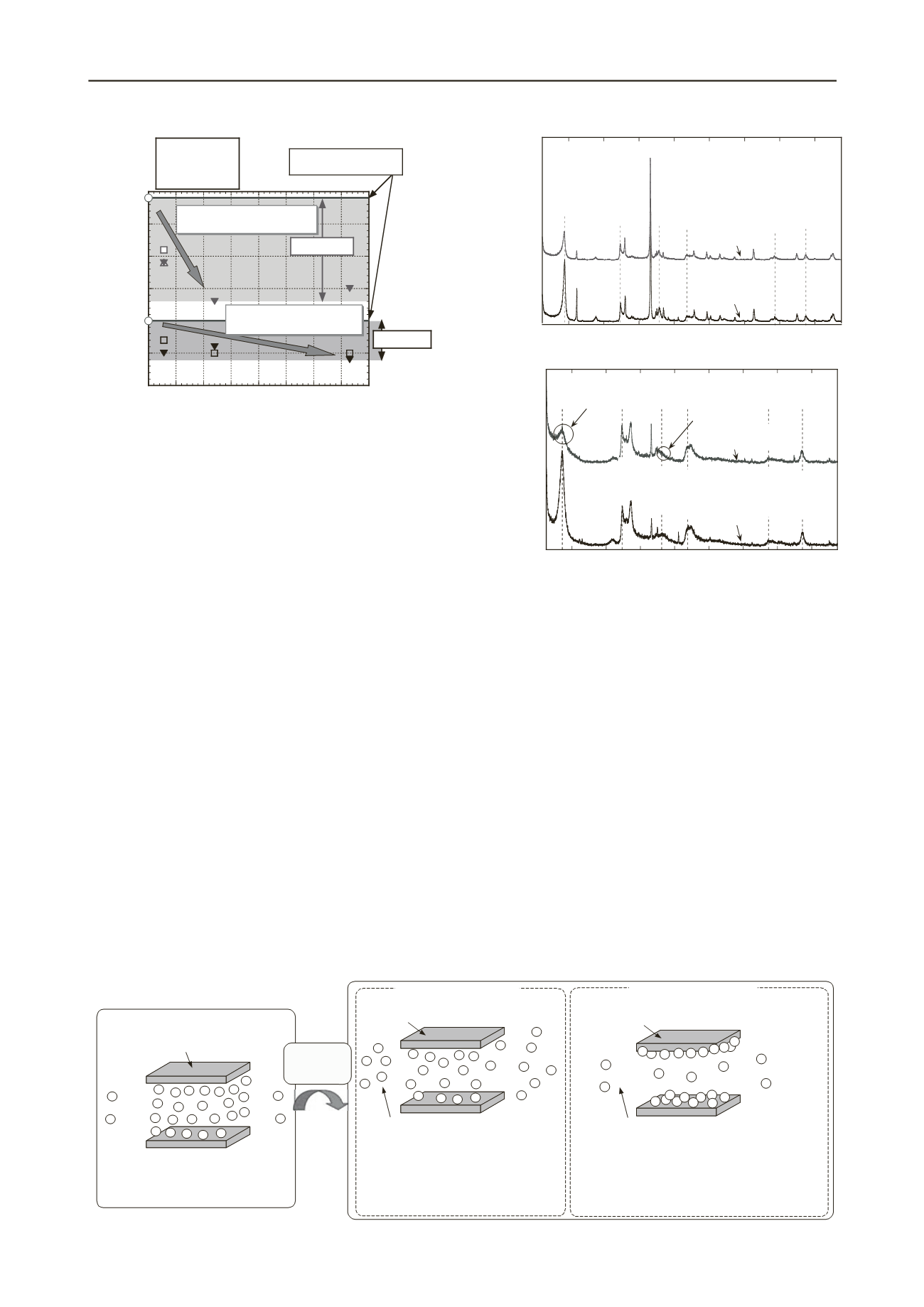
Figure 8 presents X-ray diffraction plots of bentonites A and
C experienced thermal exposure. Results show thatthe X-ray
diffraction plot of bentonite A hasalmost no change by thermal
exposure.However, the plot for bentonite C shows a marked
change because of thermal exposure. Therefore, the mechanism
of thermal effects on sodium and calcium-type bentonites can
be understood as shown in Fig. 9.
5 CONCLUSIONS
This study quantitatively assessedthermal exposure effects
onthe swelling characteristics of sodium-type and calcium-type
bentonites using swellingpressure and swellingdeformation
testing of bentonites that had undergonethermal exposure. This
reportdescribedmechanisms of thermal influences onswelling of
these heated bentonites by consideration of the experimentally
obtained results with measurements of cation concentrations of
water surrounding the specimens, with methylene blue
absorption tests, and X-ray powder method.
6 ACKNOWLEDGMENTS
This study was supported through funding by research funds of
the Japanese Ministry of Education, Culture, Sports, Science
and Technology and the TEPCO Research Foundation. The
author alsothanks all members and students of the Geotechnical
Laboratory, Ibaraki University, for their kind assistance and
discussions.
7 REFERENCES
Akagi, H. 1994. A physico-chemical approach to the consolidation
mechanism of soft clays,
Soils and Foundations
34(4), 43-50.
Japan Nuclear Cycle Development Institute (Japan Atomic Energy
Agency as of 2012) (2000).
H12: Project to establish the scientific
and technical basis for HLW Disposal in Japan
, Project Overview
Report, JNC TN1410 2000-001.
Komine, H. and Ogata, N. 1998. Thermal Influence on compacted
bentonite for nuclear waste disposal,
Proceedingsof theThird
International Congress on Environmental Geotechnics
1,39-44,
1998.
Komine, H.,Yasuhara, K. and Murakami, S. 2009. Swelling
characteristics of bentonites in artificial seawater,
Canadian
Geotechnical Journal
46(2), 177-189.
Oscarson, D. W. and Dixon, D. A. 1989.The effect of steam on
montmorillonite,
Applied Clay Science
4, 279-292.
8
16 24 32 40 48 56 64
2
(degree)
No heating
Water content 11.54%
Temperature 130 degree
Duration 365 days
Water content 12.16%
Montmorillonite, 0.150nm
Montmorillonite, 0.169nm
Montmorillonite, 0.256nm
Montmorillonite, 0.312nm
Montmorillonite, 0.448nm
Montmorillonite, 1.244nm
Bentonite A
(Kunigel-V1)
8
16 24 32 40 48 56 64
2
(degree)
No heating
Water content 17.04%
60
70
80
90
100
110
120
0 50 100 150 200 250 300 350 400
Methylene blue absorption (mmol/100g)
Heating duration (day)
○
: No heating
△
: 90 degree
□
: 110 degree
▼
: 130 degree
Bentonite A
Bentonite C
Decreasing 16 - 32 mmol/100g
by thermal influences
Decreasing 6 - 12 mmol/100g
by thermal influences
Methylene blue absorption
on condition of no heating
Figure 7.Methylene blue absorption for bentonites A and C with
thermal exposure.
1.538nm
0.450nm
0.256nm
0.170nm
0.150nm
0.310nm
Noticeable
decreasing
of the peak
No clear peak
Montmorillonite
Montmorillonite
Montmorillonite
Montmorillonite
Montmorillonite
Montmorillonite
Bentonite C
(Kunibond)
Temperature 130 degree
Duration 365 days
Water content 11.92 %
Figure 8. X-ray diffraction plots of Bentonite A and C with
thermal exposure.
+ +
+
+ +
+
+
+
+ +
+
+
+
+ +
+
+
+ + + +
+
+
+
+
+
+
Sodium-type bentonite A
Repulsive force between two montmorillonite
minerals is generated by difference of cation
concentrations between two montmorillonite
minerals and water around minerals, and swelling of
montmorillonite is caused by the above phenomena
+
Montmorillonite mineral
(Negative charge
)
+ + + + +
+
+ + +
+
+
+
+ + ++
+ + + +
+
+
+
+
+
+
Bentonite with no thermal exposure
Thermal
histories
+ + + + +
+
++
+ +
+
+
+
+ +
+
+
+ + + +
+
+
+
+
+
+
Calcium-type bentonite C
Absorbing ability of montmorillonite
is reduced by thermal history.
Cations between montmorillonite mineral layer can elute
easily to surrounding of specimen because absorbing
ability of montmorillonite is reduced.
For calcium-type bentonite C, thermal history derives to
apparently reduce absorbing ability of montmorillonite minerals by
accreting Ca
2+
and Mg
2+
to montmorillonite mineral due to
thermal history. Therefore, difference of cation concentration
between montmorillonite mineral layer and surrounding water of
specimen so swelling properties is apt to reduce.
Absorbing ability of montmorillonite is reduced because cations,
especially Ca
2+
and Mg
2+
, are accreted to montmorillonite
minerals by thermal history.
Absorbing ability of montmorillonite is apparently reduced by
accreting cations, especially Ca
2+
and Mg
2+
to montmorillonite
mineral. Therefore, cations between montmorillonite mineral layer
can not elute easily to surrounding of specimen.
For sodium-type bentonite A, thermal history derives
to reduce absorbing ability of montmorillonite
minerals and to elute cations to surrounding of
specimen from minerals. Therefore, difference of
cation concentration between montmorillonite mineral
layer and surrounding water of specimen so swelling
properties is apt to reduce.
Figure 9. Mechanism of thermal influences to bentonite-swelling


