3106
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
c) The acid soluble sulphate content is relatively low (< 0.5 %)
to a depth of 5 m, rises between 5.25 and 7 m and has a
significant peak of 4.2 % SO
4
at 7.25 m, below which it is
in the order of 0.6 % SO
4
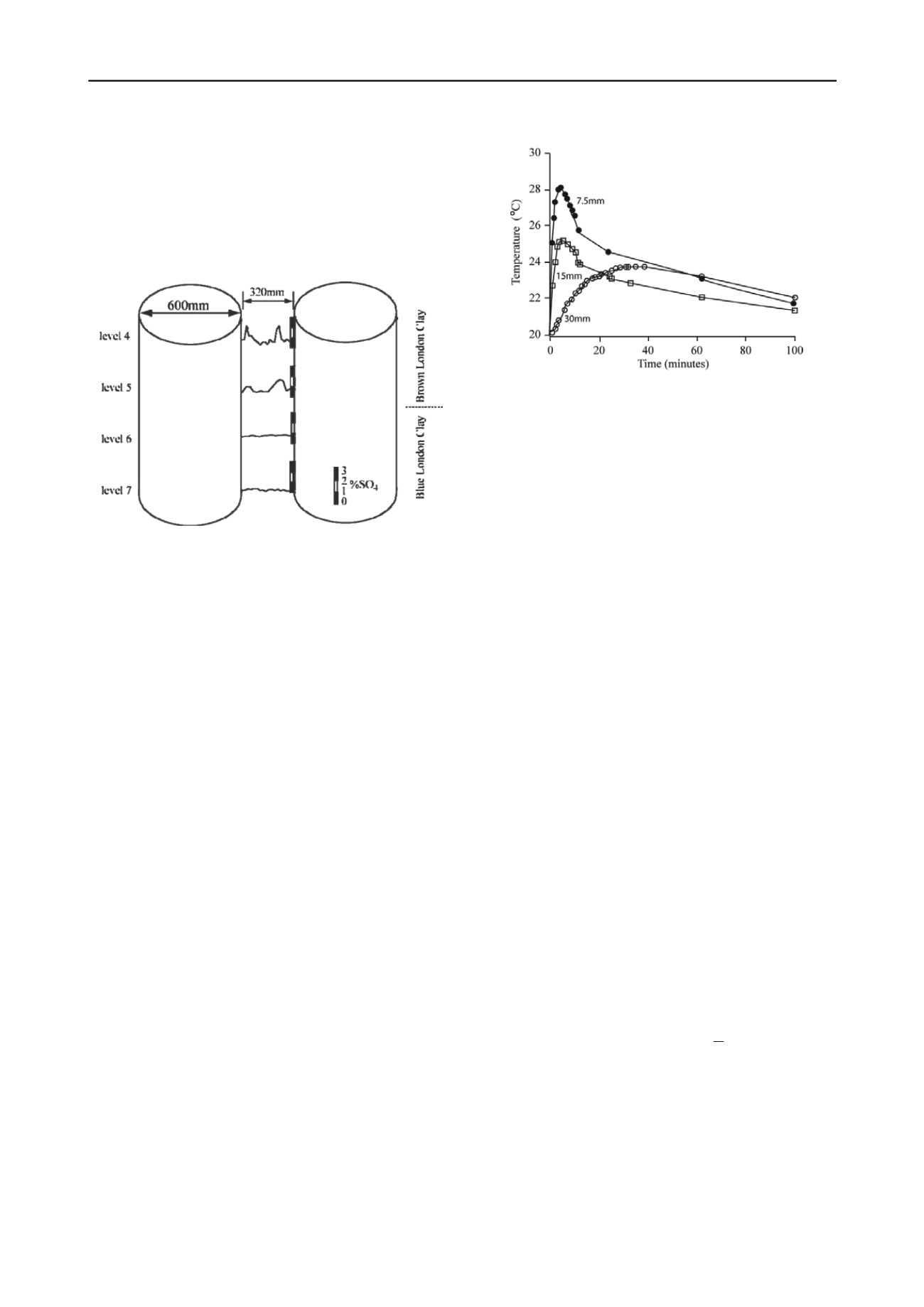
During the construction of an underground car park the
opportunity was taken to collect samples at 10 mm intervals
between two 600 mm CFA piles, 320 mm apart (Figure 6).
Figure 7. Variation in ground t
Figure 6.
SO
4
content with distance from concrete piles in the
LCF. Note variation in the brown Clay.
As noted by Hawkins and Higgins (1997), the sulphate level in
the brown LCF was generally in the order of 0.5 % SO
4
but
between the 4
th
and 5
th
floors rose to 2 %, approximately 30 mm
from each of the piles. In the underlying saturated grey LCF (6
th
and 7
th
floors) where the pyrite and calcite had not chemically
reacted, raised SO
4
values were not present. It is considered that
when the sulphates are mobilized as a consequence of the heat
of hydration, the sulphate-rich solutions move into the zone of
shear which commonly occurs in the
in situ
ground adjacent to
material affected by the auger torque.
A laboratory experiment was undertaken by Hawkins and
Higgins to determine the influence of temperature when the
LCF was heated in a moist environment at 30 °C for four, six
and eight weeks, the acid soluble sulphate rose from 0.22 to
1.32 %. This six-fold rise emphasises the effect of the heat of
hydration on the ground chemistry close to CFA piles, and
hence the care required when assessing the concrete
aggressivity class if concrete piles are to be installed in ground
containing or likely to develop sulphates.
To assess the outward migration of heat from the hydrating
concrete, Hawkins and Higgins undertook an experiment by
placing a copper pipe in an augered hole in the LCF. Three
thermometers were installed at 7.5, 15 and 30 mm from the
pipe. When water at 65 °C was placed in the copper pipe the
temperature of the Clay rose by a maximum of 8 °C in six
minutes at 7.5 mm; by 5 °C in six minutes at 15 mm and by 4
°C in 35 minutes at 30 mm (Figure 7).
emperature with time at given distances
fro a heated pipe in the LCF.
ution and abundance of these forms can
ur, as
a full suite
mination of detailed sulphate and pH profiles is
be taken to protect concrete from sulphate attack
k marcus hawkins for assistance
igures.
Ber
on: An update.
Bes
s in clay
BR
ecial Digest 1.
Building
Cha
s: the
Com
g
Haw
ations of pyrite oxidation for engineering
Haw
m
6 CONCLUSIONS
a) Sulphur is present in the LCF predominantly as pyrite and
gypsum. The distrib
be highly variable
b) Planning of site investigation works should appreciate the
spatial variation (lateral and vertical) in ground sulph
well as the type and location of sub-surface structures
c) Appropriate laboratory testing would include
of chemical testing including AS, WSS and TS
d) The deter
advocated
e) The heat of hydration of concrete may result in a
concentration of sulphates and appropriate measures should
7 ACKNOWLEDGEMENTS
The authors would like to than
in the preparation of f
8 REFERENCES
ner R.A. 1984. Sedimentary pyrite formati
Geochimica et Cosmochimica Acta
48, 605-615.
sey G.E. and Lea F.M. 1953. The distribution of sulphate
soils and groundwaters.
Proc. Inst. Civ. Eng.
2, 159–181.
E. 2005.
Concrete in aggressive ground. Sp
Research Establishment, Bracknell, UK.
ndler R.J. 2000. Clay sediments in depositional basin
geotechnical cycle.
Quart. Jnl. Eng. Geol. Hydro.
33, 7-39.
ité Technique Québécois D'étude Des Problèmes De Gonflement
Associés à La Pyrite. 2001.
Appraisal procedure for existin
residential buildings
. Procedure CTQ-M200, Version 2.0, June 4.
kins A.B. 2013. Engineering Implications of the Oxidation of
Pyrite: an overview, with particular reference to Ireland. In
Hawkins, A.B. (ed)
Implic
works
. Springer. In press.
kins A.B. and Higgins M.D. 1997. The generation of sulphates in
the proximity of cast in situ piles. In: Ground Chemistry:
Implications for Construction. A.B. Hawkins (ed). Balkema,
Haw
oor
Leg
3.
Handbook of geology in civil
Rai
an
Practice and Worldwide Trends
, Institution of Civil Engineers.
Rotterdam.
kins A.B. and Pinches G.M. 1987. Cause and significance of heave
at Llandough Hospital, Cardiff - a case history of ground fl
heave due to gypsum growth.
Quart. Jnl. Eng. Geol
. 20, 41-57.
get R.F. and Karrow P.F. 198
engineering
. McGraw-Hill.
son C.A. 1992. Deep basement construction at College Road,
Harrow. P
roceedings of the Conference on Piling, Europe


