134
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
8
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
r
= 0
v
s
= 4.0 x 10
-11
m/s
Measured-Source
Measured-Soil
D*
= 3.0 x 10
-10
m
2
/s
D*
= 5.5x 10
-10
m
2
/s
D*
= 8.0 x 10
-10
m
2
/s
0 5 10 15 20 25 30 35
Depth (m)
Bromide Concentration,
C
(mg/L)
(b)
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
0 5 10 15 20 25 30 35 40
r
= 0.60 m
v
s
= 4.0 x 10
-10
m/s
Measured-Source
Measured-Soil (
r
= 0.53 m)
Measured-Soil (
r
= 0.63 m)
D*
= 3.0 x 10
-10
m
2
/s
D*
= 5.5x 10
-10
m
2
/s
D*
= 8.0 x 10
-10
m
2
/s
Depth (m)
Bromide Concentration,
C
(mg/L)
(c)
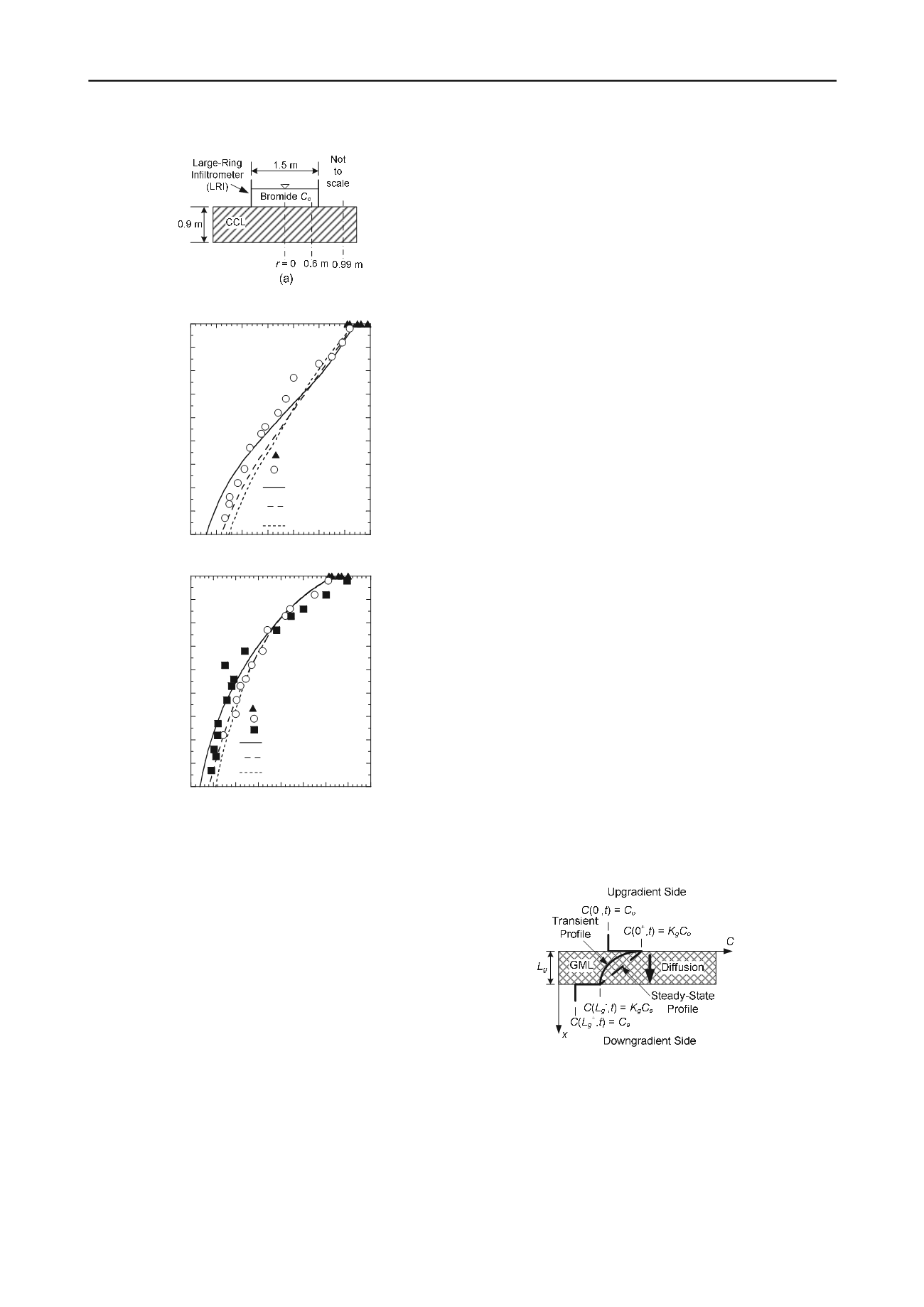
Figure 6. Bromide concentration profiles with a prototype
compacted clay liner: (a) schematic cross section of large-ring
infiltrometer; (b) and(c) concentration profiles at radii of 0 and
0.60 m, respectively, from the centerline of the LRI (modified
after Willingham et al. 2004).
4.2.3
Diffusion through Geomembrane Liners
Geomembrane liners (GMLs) are thin (typically 0.76 mm
to 3.05 mm) polymer-based materials that are commonly
used as barriers or components of barrier systems for
containment applications. In such applications, the only
way for aqueous-phase inorganic contaminants to migrate
through the polymer based GML is if the GML contains a
defect, e.g., a puncture hole or crack, or is otherwise
defective due to poor manufacturing or poor placement and
protection procedures. In such cases, the GML will offer
essentially no resistance to contaminant migration through
the defect, such that contaminant migration will readily
pass through the GML, i.e., unless the GML is founded
upon a hydraulic resistant layer, such as natural, low-
permeability clay, or the GML represents the upper
component of a composite liner which includes an
underlying low-permeability component, such as a CCL or
geosynthetic clay liner (GCL).
In the case where the GML is entirely intact, the only
way aqueous-phase contaminants can pass through the
GML is via molecular diffusion, and the only contaminants
that can diffuse substantially through the GML are those
that can partition into the polymer comprising the GML,
which generally limits the contaminants to organic
compounds, such as VOCs. For example, Rowe (2005)
reported the results of a long-term diffusion test involving
a 2-mm-thick HDPE geomembrane subjected to a
difference in NaCl concentration of 2.2 g/L, where the
measured concentration of chloride on the downgradient
side of the geomembrane after about 12 yr of exposure was
only 0.02 % of the source concentration, which was within
the range of the analytical uncertainty of the chemical
analysis. Rowe (2005) also cites the results of an
independent study that indicated negligible diffusion of
heavy metals (Cd
2+
, Cu
2+
, Mn
2+
, Ni
2+
, Pb
2+
, Zn
2+
) from a
0.5 M acid solution (pH = 1-2) through an HDPE over a 4-
yr period.
In this regard, there have been numerous studies
evaluating diffusion of a wide variety of organic chemicals
through a wide variety of different polymer-based GMLs
(Rowe et al. 1995, Park and Nibras 1996, Park et al.
1996a,b, Xiao et al. 1996, Sangam and Rowe 2001a, Joo et
al. 2004, 2005, McWatters and Rowe 2010, Jones et al.
2011, Saheli et al. 2011, Touze-Foltz et al. 2011). A
primary outcome from most of these studies is that
geomembranes formed from a single polymer, such as high
density polyethylene (HDPE),
linear low-density
polyethylene (LLDPE), very low-density polyethylene
(VLDPE), and polyvinyl chloride (PVC), typically provide
little resistance to diffusion of VOCs (e.g., Edil 2003). In
this regard, the general process for diffusion of such
organic chemicals through GMLs in response to an
aqueous-phase concentration difference, -
C
=
C
o
–
C
e
>
0, established across a GML is illustrated schematically in
Fig. 7 (e.g., see Rowe 1998, Katsumi et al. 2001). First, the
organic chemical partitions from the external aqueous
solution into the geomembrane (adsorbs) at a concentration
K
g
C
o
, where
K
g
is the chemical-geomembrane partitioning
coefficient. Second, the chemical diffuses through the
geomembrane in response to a concentration difference
within the GML of -
C
g
=
K
g
C
o
–
K
g
C
e
> 0, where
K
g
C
e
has been established on the basis of the external aqueous-
phase concentration,
C
e
. Finally, the chemical partitions
from the geomembrane (desorbs) back into the lower
bounding aqueous solution.
Figure 7. Schematic of concentration profile for organic chemical
diffusion through an intact geomembrane liner (GML) (modified
after Rowe 1998, Katsumi et al. 2001).
Since GMLs are relatively thin, steady-state diffusion
through the GML can be established relatively quickly,
such that the mass flux of the organic chemical can be
expressed in accordance with Fick's first law as follows
(Park et al. 1996a,b, Rowe 1998, Katsumi et al. 2001,
Rowe 2005):


