136
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
10
higher salt concentrations result in compression of the
adsorbed layers of cations associated with the bentonite
particles and, therefore, larger pore openings between
adjacent particles and lower
. As shown in Fig. 9b, such
larger pores due to higher salt concentrations also result in
increasing values of
D
*
for KCl with increasing
C
o
. Note
that the values of
D
*
shown in Fig. 9b are steady-state
values in that the values correspond to after steady-state
diffusion had been established with respect to both Cl
-
and
K
+
. The combined effect of
C
o
on
and
D
*
is shown in
Fig. 9c, where
D
*
is shown to decrease with increasing
such that, in the limit as
→ 1,
D
*
→ 0 as required on the
basis of the definition of a perfect or ideal membrane. As
indicated in Fig. 9b, this decrease in
D
*
with increasing
was attributed to a decrease in the apparent tortuosity
factor,
a
(see Eq. 1).
0
0.2
0.4
0.6
0.8
1
0.001
0.01
0.1
(a)
Membrane Efficiency
Coefficient,
Source KCl Concentration,
C
o
(M)
= -0.457 - 0.455log(
C
o
)
(r
2
=0.998)
0
1
2
3
0.001
0.01
0.1
(b)
Effective Diffusion Coefficient,
D*
(x 10
-10
m
2
/s)
Source KCl Concentration,
C
o
(M)
0
1
2
3
0
0.1
0.2
0 0.2 0.4 0.6 0.8 1
(c)
a
Apparent Tortuosity Factor,
a
Membrane Efficiency Coefficient,
a,max
= 0.12
D
*
= 2.4 x 10
-10
m
2
/s
Effective Diffusion Coefficient,
D
*
(x 10
-10
m
2
/s)
D
*
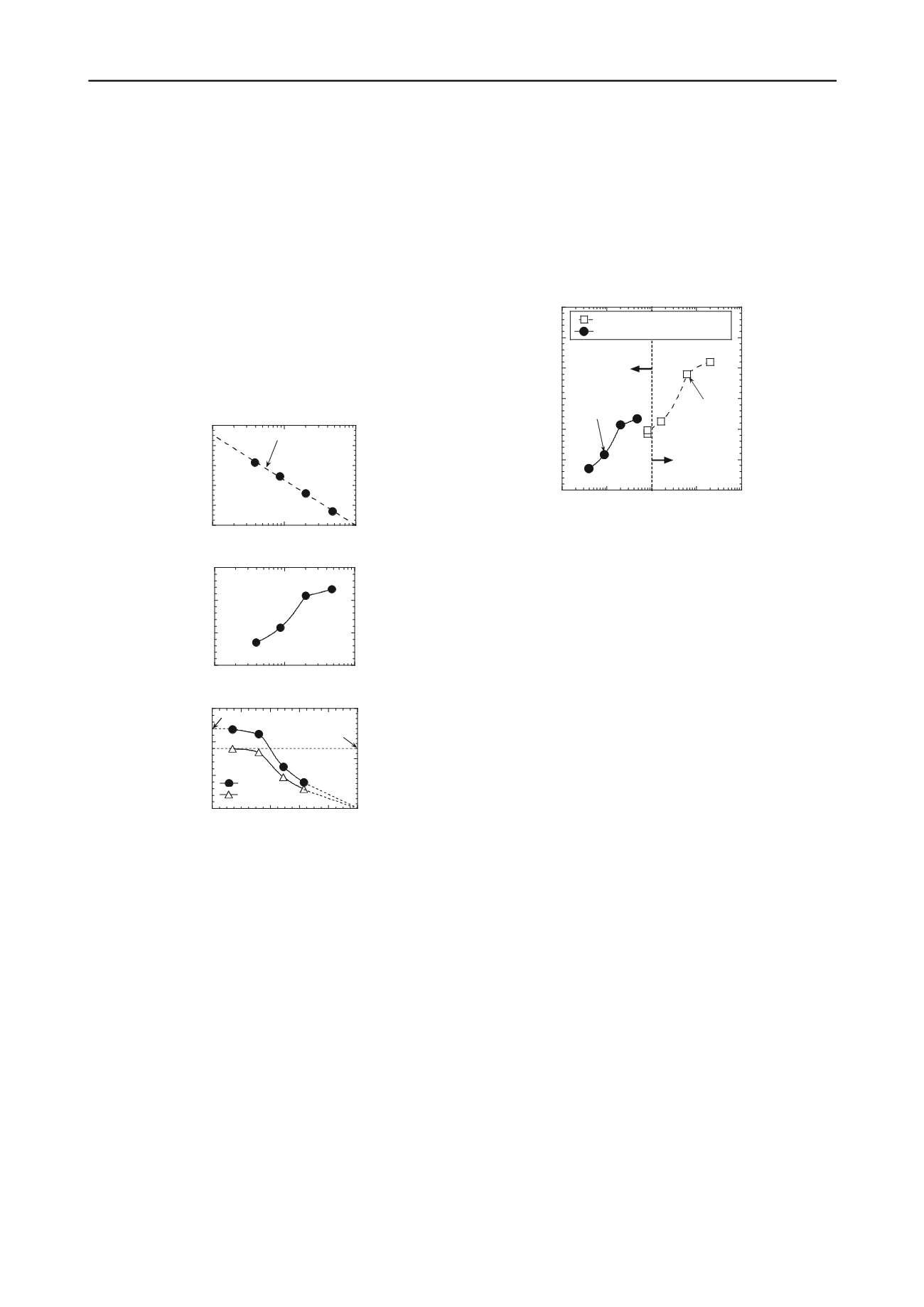
Figure 9. Results of a test to measure simultaneously the diffusion
of KCl through a GCL and the membrane behavior of the GCL:
(a) membrane efficiency of the GCL; (b) steady-state diffusion
coefficient of KCl; (c) effect of membrane behavior on steady-
state diffusion of KCl (modified after Malusis and Shackelford
2002a,b).
Malusis and Shackelford (2002a) compared their results
with those reported by Lake and Rowe (2000) based on
measurement of NaCl diffusion under constant volume
conditions through granular sodium bentonite extracted
from a GCL. The results of this comparison are shown in
Fig.10 in the form of the
D
*
values for KCl and NaCl
versus the source salt concentration,
C
o
. Overall, results in
Fig. 10 indicate a similar trend of increasing
D
*
with
increasing
C
o
. Although
values were not measured by
Lake and Rowe (2000), chemico-osmotic flow was
reported to be sufficiently negligible such that the authors
concluded that membrane behavior probably wasn't
significant for the range of NaCl concentrations used (i.e.,
C
o
≥
0.08 M). The superimposed demarcation between
membrane behavior (
> 0) and no membrane behavior (
= 0) based on the results shown in Fig. 9 tends to support
this conclusion, although the relationship between
and
C
o
for the granular bentonite used by Lake and Rowe
(2000) may not be the same as that shown in Fig. 10 due,
in part, to the different porosity of the specimens (
n
= 0.78
to 0.80 vs.
n
= 0.71), different salts used in the tests (KCl
versus NaCl), and the potentially different properties of the
granular bentonites in the two GCLs. Despite these
differences, the results shown in Fig. 10 suggest that there
is general agreement between the results reported in the
two studies.
0
1
2
3
4
5
6
0.001 0.01
0.1
1
10
Lake & Rowe (2000)
Malusis & Shackelford (2002a)
Effective Diffusion Coefficient,
D*
(x 10
-10
m
2
/s)
Source Salt Concentration,
C
o
(M)
KCl
(
n
= 0.78 - 0.8)
NaCl
(
n
= 0.71)
Membrane
Behavior
(0 <
< 1)
No
Membrane
Behavior
(
= 0)
Figure 10. Comparison of the results for the diffusion of salts
through GCLs from two different studies (modified after Malusis
and Shackelford 2002a).
4.2.5
Diffusion through Composite Liners
Composite liners refer to engineered barriers that are
comprised of more than one type of barrier in intimate
contact with each other. Although there are a variety of
possible composite liner systems, including those that
contain more than two component types of barriers (e.g.,
Nguyen et al. 2011), the most common types of composite
liners consist of a GML overlying and in intimate contact
with either an underlying CCL or an underlying GCL,
although other composite liner scenarios are possible. For
these common composite liners, the effectiveness of the
composite liner in restricting contaminant migration relies
largely on the integrity of the overlying GML and on the
intimacy of the contact between the overlying GML
relative to the underlying CCL or GML (Rowe 1998,
Foose et al. 2001, 2002). The fewer the number of defects
in the GML and the more intimate (tighter) the contact
between the two barriers, the more effective the barrier in
restricting contaminant migration. However, failure to
protect the GML could compromise the integrity of the
composite liner.
For example, Rowe et al. (2003) evaluated the
performance of a composite liner comprised of a 1.5-mm-
thick HDPE GML overlying a 3-m-thick CCL after 14
years in operation as a leachate lagoon liner (also see
Rowe 2005). The GML had been poorly protected,
resulting in development of 528 defects (cracks, holes,
patches) per hectare over the 14-yr operational life of the
liner, which allowed leachate to seep between the GML
and CCL. Data obtained upon decommissioning indicated
that leachate leaking through the GML had spread quickly
over the entire interface between the GML and CCL,
essentially rendering the GML ineffective. However, there
were questions as to when the GML became ineffective as
a barrier component and to what extent contaminant had
penetrated the underlying CCL. Based on these
considerations, Rowe et al. (2003) evaluated the chloride
concentration profile within the CCL based on samples
recovered from five different locations. As illustrated in


