135
Honour Lectures /
Conférences honorifiques
9
o e
d g g
g
C C
J D K
L
(6)
where
D
g
is the diffusion coefficient for the chemical in the
GML. Conservative (high) estimates of
J
d
will occur when
C
e
is assumed to be zero.
Since geomembranes are not porous media, the nature
of
D
g
is not the same as that of
D
*
. For example, based on
an extensive summary of both
K
g
and
D
g
values from the
literature reported by Rowe (1998), the upper limit on the
vast majority of the
D
g
values is on the order of 1 x 10
-1l
m
2
/s, with numerous values ranging from one to several
orders of magnitude lower than this value. Thus, values of
D
g
generally are several orders of magnitude lower than
values of
D
*
. However, despite such low magnitude
D
g
values, Park et al. (1996b) illustrate that molecular
diffusion of organic chemicals through intact GMLs can be
substantially greater than leakage through geomembrane
defects. A major reason for this difference is that that
cross-sectional area for diffusive mass flux through a GML
is the entire surface of the GML, whereas mass flux due to
leakage through a GML is associated with only a small
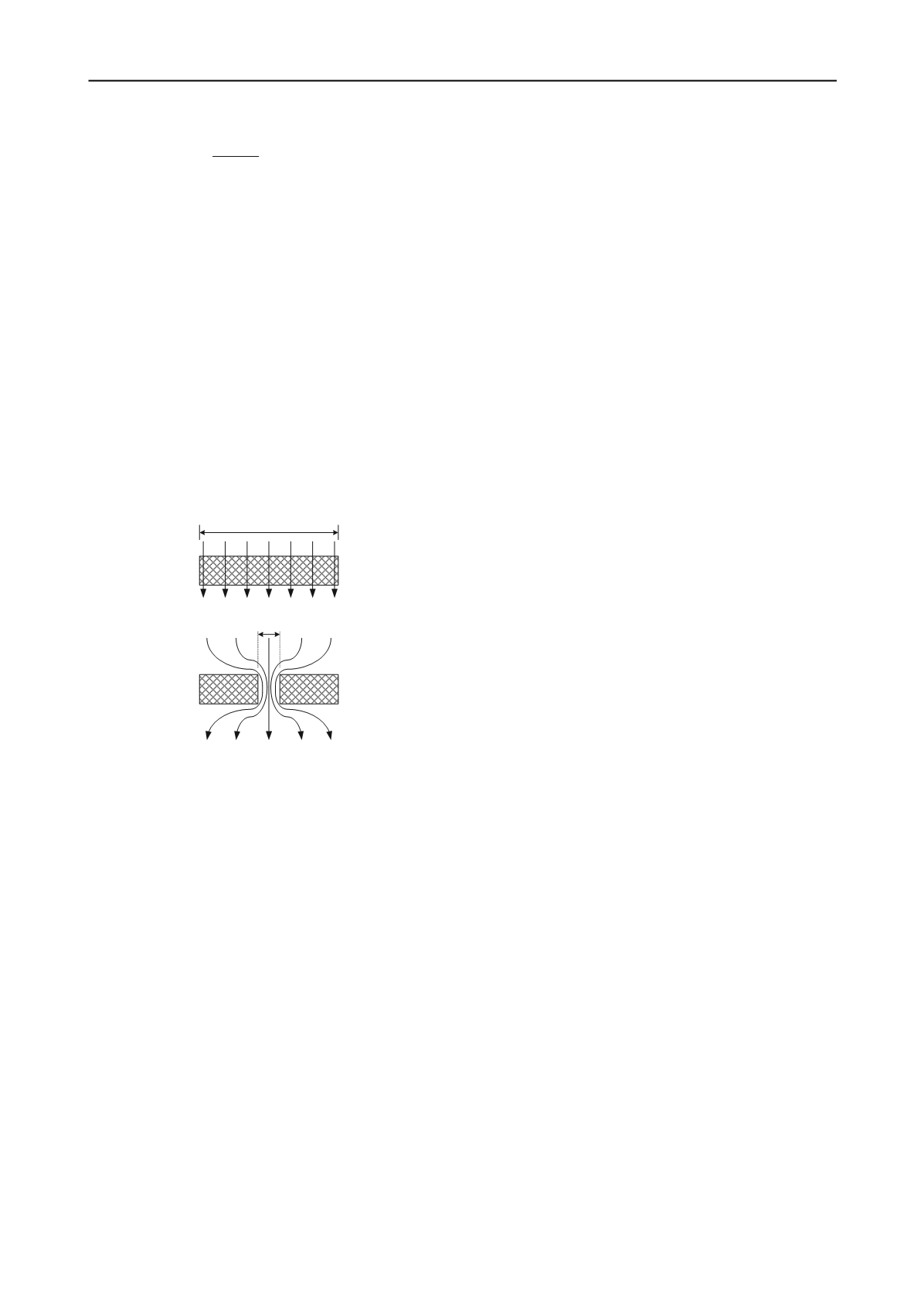
percentage of the surface area (see Fig. 8).
Leakage through Defect Area,
A
d
Area,
A
Diffusion
Figure 8. Cross-sectional areas for diffusion versus leakage
through a GML.
Because diffusion of VOCs through single polymer
GMLs has been an issue, recent research has focused on
evaluating alternative GMLs for the ability to minimize
VOC diffusion. For example, Sangam and Rowe (2005)
evaluated the effect of fluorinating the surface of an HDPE
on the diffusion of VOCs through the GML. In essence,
the surface fluorination reduces the affinity of the GML to
VOCs. Sangam and Rowe (2005) reported that the
diffusion coefficient for the surface fluorinated HDPE was
on the order of 1.5 to 4.5 times lower than that for the
untreated HDPE, depending on the specific hydrocarbon
evaluated. Similarly, McWatters and Rowe (2010)
evaluated the ability of two coextruded GMLs to reduce
the diffusive flux of VOCs. Coextrusion involves
extruding two or more layers of dissimilar polymers into a
single film. McWatters and Rowe (2010) reported
improved resistance to BTEX diffusion for the two
coextruded GMLs, a polyamide (nylon) GML and an
ethylene vinyl-alcohol (EVOH) GML, relative to that for
either an LLDPE or a PVC GML. The results of these and
other studies indicate that alternatives to the single
polymer GMLs may offer improved performance in terms
of VOC diffusion.
4.2.4
Diffusion through Geosynthetic Clay Liners
Geosynthetic clay liners (GCLs) are relatively new barrier
materials, having first been used in a landfill in 1986
(Bonaparte et al. 2002). Traditional or conventional GCLs
are thin (~ 5 to 10 mm), prefabricated (factory
manufactured) hydraulic barriers (liners) that consist
primarily of a processed clay, typically sodium bentonite,
or other low permeability material that is either encased or
"sandwiched" between two geotextiles or attached to a
single polymer membrane (i.e., geomembrane) and held
together by needle-punching, stitching, and/or gluing with
an adhesive. The hydraulic resistance of these conventional
GCLs that do not include a geomembrane or polymer film
is attributed to the bentonite component of the GCL, which
swells in the presence of water to form a tight sealing
layer. Although GCLs can be subjected to significant
incompatibility upon permeation with chemical solutions
or liquids, resulting in potentially significant increases in
hydraulic conductivity, the values of
k
h
for GCLs
permeated with dilute chemical solutions or water tend to
be less than about 1 x 10
-10
m/s (e.g., Shackelford et al.
2000). Such low
k
h
values and the relative thinness of
GCLs imply that diffusion would be a significant, if not
dominant, transport process through GCLs. Accordingly,
several studies have evaluated the diffusion of chemicals
through GCLs (Lake and Rowe 2000, 2005, Rowe et al.
2000, Malusis and Shackelford 2002a, Lange et al. 2009,
Paumier et al. 2011, Malusis et al. 2013).
For example, consider the results of the study shown in
Fig. 9 for diffusion of KCl through a GCL. In this study,
diffusion of KCl was hypothesized to be affected by the
ability of the bentonite in the GCL to exhibit
semipermeable membrane behavior, whereby solutes are
excluded from the smaller pores in the clays, thereby
restricting the diffusion of the KCl (Malusis and
Shackelford 2002b). Such solute restriction also results in
chemico-osmosis, or the movement of liquid from lower
solute concentration to higher solute concentration, or
opposite to the direction of diffusion. Accordingly, the
GCL was tested in an apparatus that was able to measure
simultaneously both the membrane efficiency of the GCL
and the
D
*
of the KCl.
The membrane efficiency refers to the relative degree
or extent of solute restriction (also referred to as "ion
exclusion"), and is quantified in terms of a membrane
efficiency coefficient,
(Shackelford et al. 2003).
Although negative values of
have been reported in some
cases due to atypical circumstances resulting from
processes such as "diffusion-osmosis" (Olsen et al. 1990),
values typically range from zero for clays exhibiting no
membrane behavior and, therefore, no solute restriction, to
unity (100 %) for "perfect" or "ideal" membranes that
restrict the passage of all solutes. Because soils generally
exhibit a range of pore sizes, some of the pores in clays
may be restrictive whereas others are not. As a result, most
natural soils that exhibit membrane behavior do so as
"imperfect" or "non-ideal" membranes, such that 0 <
< 1
(Shackelford et al. 2003). In particular, bentonite has been
shown to possess the potential for significant membrane
behavior, such that the possible effect of membrane
behavior on solute transport through any bentonite-based
barrier should be considered (Shackelford 2011, 2012,
2013).
In terms of the results in Fig. 9, Fig. 9a shows the
correlation between the measured value of
for the GCL
and the source concentration of KCl,
C
o
, used in the test.
Due to physico-chemical interactions between the salts in
the pore water of the bentonite and the bentonite particles,


