2554
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
agglomerates. In other words, only part of the porewater
contributes to plasticity. This is similar to diatoms with
poriferous particles in soils such as the Mexico City clay, and
andosols containing allophane in which water is trapped within
soil aggregates (Mesri et al. 1975, Terzaghi et al. 1996). Both
soils display unusually high plastic limits. In summary, total
lime content, l
c
, is used up through adsorption, l
ca
, and
dissolution, l
cd
.
The time-dependent manifestation of adsorbed lime is a
gradual chemical reaction of calcium hydroxide with soil
particle surfaces. As the reaction products continue to form and
later harden or crystallize at the reaction sites of adsorbed lime
particles, they improve soil particle connections within the flocs
and agglomerates that may mature into porous soil aggregates
(Baver 1956). The proposed concept of lime particle adsorption
on soil particles is somewhat similar to physical adsorption of
calcium hydroxide molecules proposed by Diamond and Kinter
(1965). However, considering that a clay-sized hydrated lime
particle may contain 10
11
molecules of Ca(OH)
2
, a more
significant time-dependent chemical reaction of adsorbed lime
with soil particle surfaces is expected for adsorbed lime particles
than for adsorbed lime molecules. Richardson et al. (1994) have
mentioned layers of Ca(OH)
2
sandwiched between silicate
layers
.
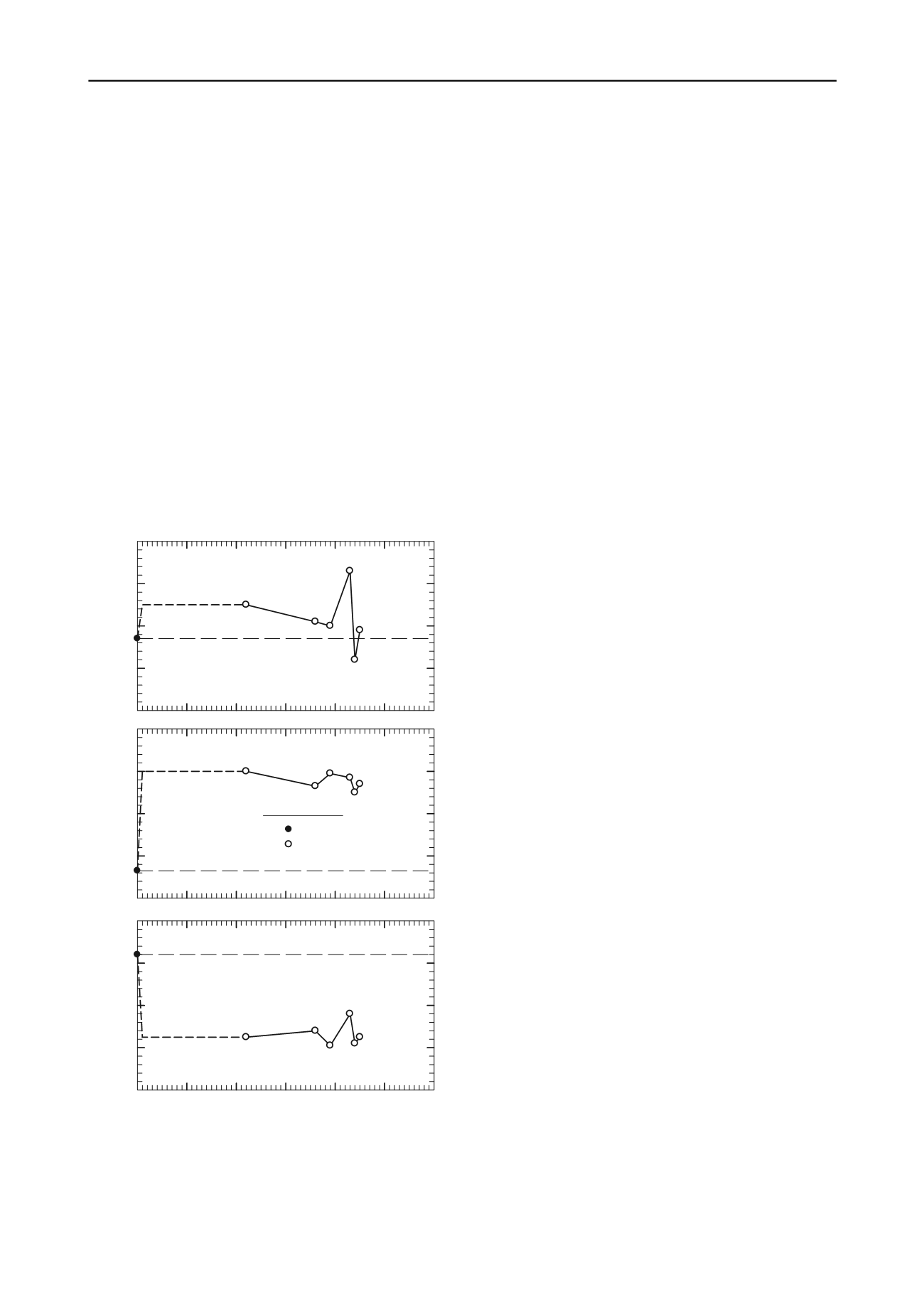
100
110
120
130
140
Untreated
Water Content, %
20
40
60
80
100
0
6.6
Untreated
Curing Time, days
0
10
20
30
40
50
60
20
40
60
80
100
Untreated
Liquid Limit
Plastic Limit
Plasticity Index
Lime Content, %
Figure 1. Lime-Brenna clay interaction under effective confining
pressure
The lime content required to fully satisfy adsorption is
mainly related to soil particle size and shape and therefore, the
mineralogy of soil solids (Goldberg and Klein 1952, Eades and
Grim 1960) and degree of dispersion or aggregation. As soil
particle size decreases and therefore, surface area increases, l
ca
increases. Lime content consumed through adsorption is
probably also related to the soil water content as it influences
dispersion of soil particles and facilitates thorough mixing to
allow full distribution and intimate contact between lime and
soil particles, degree of pulverization of hydrated lime, and the
intensity of mixing.
Because the solubility of calcium hydroxide in water is very
small, for typical soil water contents a very small lime content is
required to saturate the porewater. However, experience
indicates that pH remains below 12.3-12.4 for lime contents far
in excess of that required for the saturation of porewater. This
behavior appears to suggest that lime adsorption must be
satisfied before lime is dissolved in the porewater to increase the
pH. Zolkov (1962) considered it as remarkable that in spite of
the very small solubility of lime in water, large amount of lime
was required "to bring the pH of the soil slurry to 12.6."
Most of the chemical reaction products have a layer
structure, have high surface area, and a particle morphology that
has been described as thin plates, foils, and rolled up sheets
(Diamond et al. 1964; however sometimes fibers or laths occur
which could contribute to particle interlocking, Richardson et al.
1994). On the other hand, adequate but not excessive lime attack
may improve morphology of existing soil particles by producing
ragged, irregular, frosted or serrated particles and following
proper compaction connect them by the new reaction products.
These features are expected to improve mechanical behavior of
soils.
Because some of the reaction products during the
stabilization process are amorphous and hydrated, drying of
lime-treated soils during stabilization is likely to result in some
irreversible dehydration as well as irreversible aggregation.
3 BRENNA FORMATION
The highly plastic lacustrine clays of Lake Agassiz lead to slope
instability along the banks of the Red River that separates Grand
Forks, North Dakota from East Grand Forks, Minnesota, as it
flows north to Lake Winnipeg in Manitoba, Canada (Mesri and
Huvaj 2004). The clays of the Red River slopes are the glacio-
lacustrine deposits of glacial Lake Agassiz that is believed to
have existed from 13,000 to 8,500 years before present, during
the Late Wisconsin Glacial Episode of the Pleistocene Epoch
(Quigley 1980).
The Brenna Formation, which is characterized as a uniform,
soft to firm, dark grey, glacio-lacustrine clay with little or no
visible stratification, is full of slickensided surfaces. The major
source of sediment for the Brenna Formation was the highly
plastic montmorillonitic Pierre Shale bedrock (Quigley 1968,
Baracos 1977). The clay size fraction of Brenna Formation
ranges from 60 to 95% (Arndt 1977). This unit is divided into
Lower Brenna and Upper Brenna members. The natural water
content, plastic limit and liquid limit of Lower Brenna are in the
range of 42 to 69%, 20 to 40%, and 62 to 103%, respectively,
and the corresponding range for Upper Brenna are 60 to 85%,
27 to 38%, and 107 to 154%, respectively. Samples of both
Lower Brenna and Upper Brenna were used in the present
investigation.
4 TESTS ON LIME-TREATED BRENNA CLAY
Drained direct shear tests on lime-treated Brenna clay were
performed using reconstituted specimens. Drained multiple
reversal direct shear tests on precut specimens were used to
measure residual shear strength, and drained direct shear tests
on uncut specimens were used to measure fully softened shear
strength. Air dry Brenna clay was pulverized until all of a
representative sample passed the no. 200 US standard sieve.


