2492
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
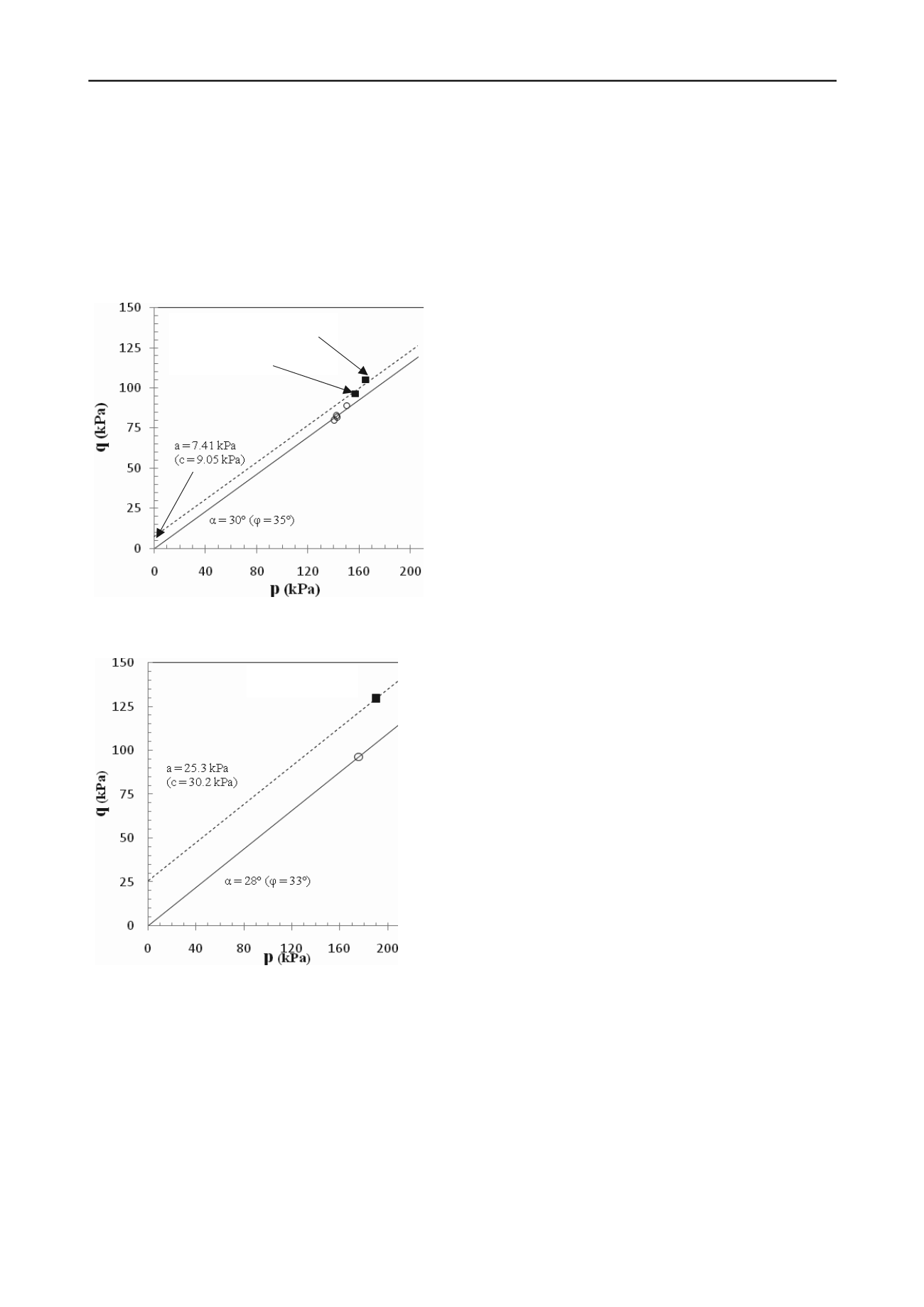
of the triaxial compression tests performed on the 20-30 Ottawa
sand are presented in Figure 3 and the results for the F-60
Ottawa sand are presented in Figure 4. The carbonate cement
content for one of the 20-30 silica sand columns was 2.0%
CaCO
3
(by weight). The carbonate content of the other 20-30
Ottawa sand column could not be quantified due to unintended
sample loss. The carbonate cement content for the finer grained
F-60 Ottawa sand was 1.6% CaCO
3
(by weight). The results
show substantial strength increase for all 3 sand columns tested.
Figure 3. p-q plot failure envelopes for 20-30 silica sand:
■
Cemented
(D
r
= 60%);
○
Uncemented (D
r
= 60%)
Figure 4. p-q plot failure envelopes for F-60 silica sand:
■
Cemented (D
r
= 35%);
○
Uncemented (D
r
= 37%);
4. CONCLUSION
Sand column tests at Arizona State University have shown that
agriculturally-derived urease can be used to induce calcium
carbonate precipitation in sand. Sand columns were developed
using Ottawa 20-30 and F-60 sand and three different
preparation methods: dry pluviation followed by percolation of
a calcium-urease-urea cementation solution, pluviation into a
calcium-urease-urea cementation solution, and mixing the sand
with urease prior to pluviation with a calcium-urea solution.
Cementation was observed in all of the columns. XRD and
SEM testing confirmed that calcium carbonate (specifically
calcite) was the cementing agent. Acid digestion showed that
increased applications yielded correspondingly greater
carbonate precipitation. The quality of cementation, as
determined by the effort needed to break apart cemented chunks
of sand, varied depending on the sampling location within the
column. Triaxial test results on cemented columns showed
substantial strength increase over non-cemented columns at the
same relative density.
5. REFERENCES
Baumert, K.A., Herzog, T., Pershing, J., 2005
.
Navigating the Numbers
Greenhouse Gas Data & International Climate Policy
World
Resources Institute
2.0% CaCO
3
Blakely, R.L. and Zerner, B., 1984. Jack Bean Urease: The First Nickel
Enzyme.
Journal of Molecular Catalysis
23, 263–292.
CaCO
3
not quantified
Burbank, M., Weaver, T., Lewis, R., Williams, T., Williams, B. and
Crawford, R. 2012. Geotechnical Tests of Sands Following
Bio-Induced Calcite Precipitation Catalysed by Indigenous Bacteria
ASCE JGGE
132, DOI:
10.1061/(ASCE)GT.1943-5606.0000781.
Chou, C.W., Seagren, E.A., Aydilek, A.H., and Lai, M. 2011.
Biocalcification of Sand through Ureolysis.
JGGE
137, 1179–1189.
Ciurli S., Marzadori C., Benini S., Deiana S. and Gessa C., 1996.
Urease from the soil bacterium Bacillus pasteurii: Immobilization on
Ca-polygalacturonate.
Soil Biol. & Biochem
. 28, 811-817.
Das, N., Kayastha, A.M. and Srivastava, P.K., 2002. Purification and
characterization of urease from dehusked pigeonpea (Cajanus cajan
L.) seeds.
Phytochemistry
61 (5), 513-521.
DeJong, J.T., Fritzges, M.B., and Nusslein, K. 2006. Microbially
Induced Cementation to Control Sand Response to Undrained
Shear
. ASCE JGGE
132, 1381–1392.
DeJong, J.T., Mortensen, B.M., Martinez, B.C., and Nelson, D.C., 2010.
Bio-mediated soil improvement.
Ecological Engineering
197-210.
Harkes, M. P., van Paassen, L. A., Booster, J. L., Whiffin, V. S., and
van Loosdrecht, M. C. M. 2010. Fixation and distribution of
bacterial activity in sand to induce carbonate precipitation for
ground reinforcement.
Ecological Engineering
36 (2), 112–117.
Karatas, I., 2008. Microbiological Improvement of the Physical
Properties of Soils. PhD. Dissertation, Department of Civil,
Environmental, and Sustainable Engineering Arizona State
University, Tempe, AZ.
1.6% CaCO
3
Kavazanjian, E. and Karatas, I., 2008. Microbiological Improvement of
the Physical Properties of Soil
, 6
th
International Conference on
Case Histories in Geotech. Eng.
, Arlington, VA August 11-16.
Krogmeier, M.J., McCarty, G.W. and Bremner, J.M., 1989.
Phytotoxicity of foliar-applied urea.
Proc.
Natl. Acad. Sci.,
USA
86, 8189–8191.
Marzadori, C., Miletti, S., Gessa, C. and Ciurli, S., 1998.
Immobilization of jack bean urease on hydroxyapatite: urease
immobilization in alkaline soils.
Soil Biol. & Biochem
. 30 (12),
1485-1490.
Pettit N. M., Smith A. R. J., Freedman R. B. and Burns R. G., 1976.
Soil urease: activity, stability, and kinetic properties.
Soil Biol. &
Biochem
. 8, 479-484.
Srivastava, P.K. and Kayastha, A.M., 2001. Characterization of gelatin-
immobilized pigeon pea urease and preparation of a new urea
biosensor.
Biotechnology and Applied Biochemistry
34, 55-62.
van Paassen, L.A., Ghose, R., van der Linden, T.J.M., van der Star,
W.R.L., and van Loosdrecht, M.C., 2010. Quantifying Biomediated
Ground Improvement by Ureolysis: Large-Scale Biogrout
Experiment.
ASCE JGGE
136, 1721–1728.
van Paassen, L.A., Daza, C.M., Staal, M., Sorokin, D.Y. and van
Loosdrecht, M.C., 2008. In situ soil reinforcement by microbial
denitrification.
1st Int. Conf. on Bio-Geo-Civil Engineering
,
Netherlands, 124-133, June 23-25.
Whiffin, V.S., van Paassen, L.A., and Harkes, M.P., 2007. Microbial
Carbonate Precipitation a Soil Improvement Technique.
Geomicrobiology
24, 1-7.


