412
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
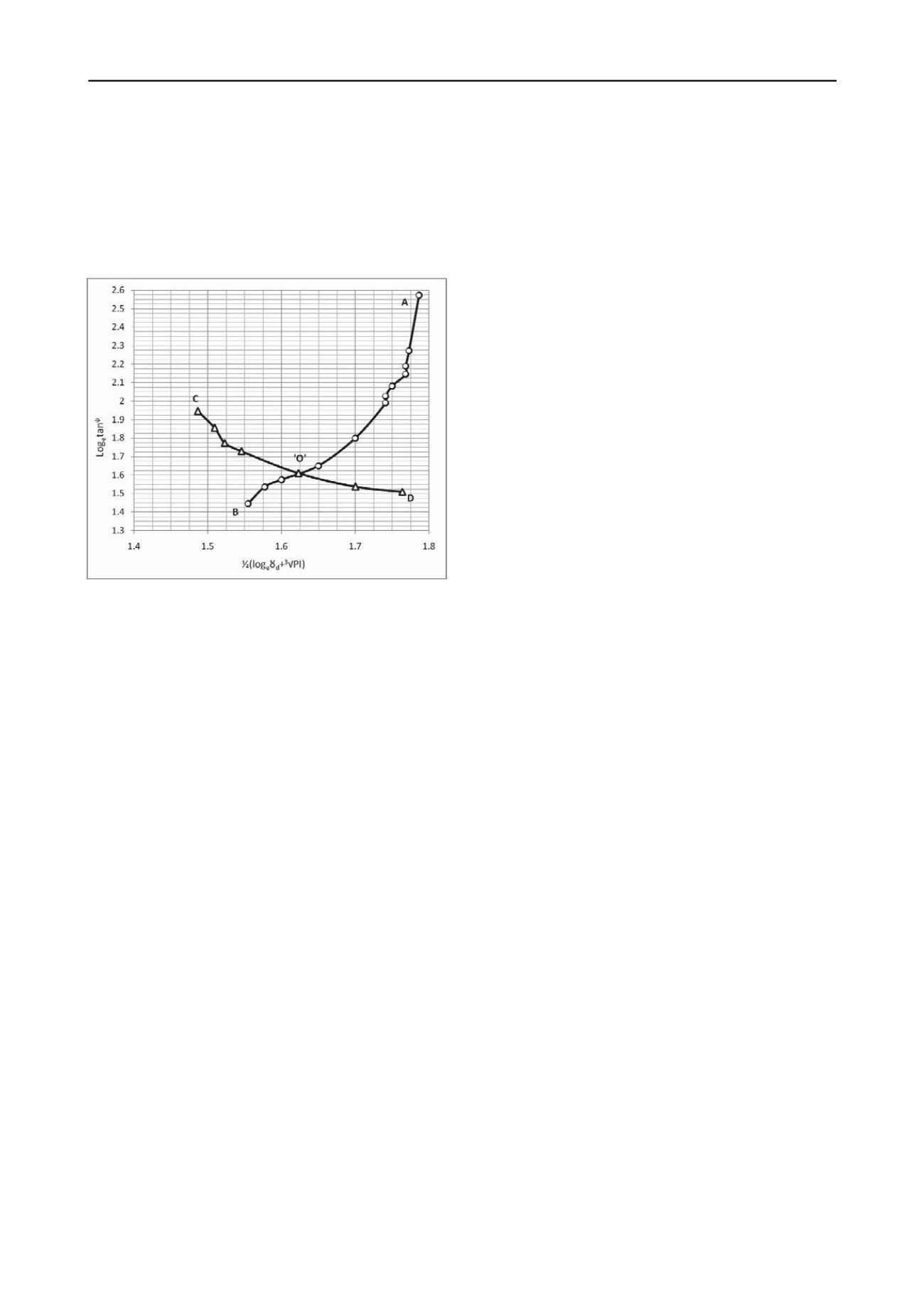
model holds good for the parameters of other sites of the region.
Accordingly, supplementary test-data were obtained from the
samples of various sites, where investigations were undertaken
by the Road Research Laboratory (RRL). Based on the data, so
obtained from RRL, values of the two distinguished factors as
shown in the abscissa and ordinates of Fig 1 were plotted and
were found corroborating with the model. This simulation
exercise had encouraged further investigations on the
interpretation of data and development of correlation, exploring
various geochemical and other technical evidences in context to
the two classified characteristics of the model.
Figure 1. The constitutive model of elastic and index properties of
cohesive soil distinguished by geological characteristics
3 INTERPRETATION
The constitutive model, as shown in Fig 1, was required to be
interpreted with severe setbacks invading it. However, the
expectation that the engineering characteristics of soil depend
on the geological properties was given due attention.
Accordingly, for clayey soil it had been the expectation that the
values of PI, γ
d,
and ψ were under the profound influence of the
texture, structure, composition, and other geological parameters.
A most common form of geo-chemical test was carried out
and found to be of much help in interpretation of the parameters
that influence the nature of the graphical model.
The ultimate extent of interpretation was to investigate how
the engineering properties of the clays of the study area have the
bearing on physical, chemical, and biological weathering
processes of rock and how such information on geological
variations of the soil samples could be correlated for estimation
of the elastic settlement of structures.
4 CORRELATION
Correlation among the various geological and engineering
properties was found to be a complex process, which
nonetheless was attempted within the limited scope of this
research through geochemical analysis. The samples were
identified as medium to highly plastic inorganic clay of semi-
pervious to impervious nature. This identification process was
based on the results obtained from the laboratory tests carried
out to investigate the engineering properties of the samples
under study.
The study samples were predominantly Kaolinite and Illite
(formed by decomposition of Potash Feldspar), Biotite (mostly
altered to Chlorite and Serpentinite), partly weathered Quartz,
and possibly Montmorillonite. Although the exact crystal
structure of clay minerals could not be known in thin slides,
nonetheless, geochemical and other indirect evidences proved
these to be clays obtained predominantly by the decomposition
of Feldspar.
5 GEOCHEMICAL EVIDENCE
The mineralogy of sedimentary rocks were characterised by two
distinct types of minerals, first, the resistant mineral obtained
from the mechanical breakdown of the parent rock, and, second,
the minerals newly formed from the products of chemical
decomposition. The latter minerals were generally hydrated
compounds.
"Goldich (1938) pointed out that the order of the
stability of minerals of igneous rock towards weathering is the
reverse of their order in the reaction series of Bowen (1915 a &
b)"
- Mason and Moore 1991. The identity of arrangement
between Bowen’s reaction series and Goldich’s stability series
indicates that the last-formed minerals of igneous rock are more
stable in subtropical temperature than the minerals formed at an
early stage of crystallisation. In other words, the difference
between the conditions at the time of formation and those
existing at the surface reflects the order of stability of common
silicate of igneous rocks.
Quartz and Feldspar are the abundant and dominant
minerals. Whereas Quartz is very resistant to the chemical
attack, feldspar is less resistant under identical scenario.
Although Feldspar may persist indefinitely in sedimentary
rocks, they are chemically decomposed by prolonged
weathering. In particular, Feldspars give rise to clays with
Potash Feldspar reacting in the presence of water to give Illite
and Plagioclase Feldspar reacting in a similar manner to give
Montmorillonite.
The samples under study were predominantly a mix
structure of Kaolinite and Illite with the presence of Ferro-
magnesium minerals and weathered Quartz. However, the
presence of Montmorillonite was doubtful as because the
studied samples did not show the typical expansive
characteristics.
The specific gravity is generally low when rock contains
light coloured minerals like Quartz and Feldspar and is high
when rocks contain dark coloured minerals, for example,
Ferromagnesian. However, clay minerals generally have a mean
specific gravity value of about 2.7, but the samples that were
studied showed a range of values of specific gravities from 1.99
to 2.65. It was expected that the samples would contain some
amount of organic matter and possibly more decomposed
Feldspar than decomposed Biotites as they are more resistant
than Feldspar or other Ferromagnesian minerals. The only
exception being the sample which lies at the point of
intersection between two curves of the model.
6 INDIRECT EVIDENCE
The clay sample of the study area typically Quaternary deposits,
which were the derivation of the Precambrian Granite and
Quartzo Feldspathic Gneisses, had been influenced by all three
types of weathering processes, namely, physical, chemical, and
biological, of which chemical is dominant.
The Precambrian Granitic rock, which includes the Quartzo
Feldspathic Gneisses of the area, are composed of
predominantly Quartz, Feldspar, and Biotite as primary
minerals. Therefore, it is obvious that decomposition of these
minerals have led to the formation of clays. Since Quartz and
partly Biotite are resistant to chemical weathering, the role of
Feldspar stands out in this regard. The Feldspar easily
decomposes in the presence of rain water and in presence of
carbon-dioxide in atmosphere. The product of the
decomposition is clay which plays an important role in the
formation of soil of the study area. Quartz remains unchanged
in the process of chemical decay and therefore presence of some
amount of silt and also sand at depth greater than 15m is
notable. Biotite on decomposition yields yellowish clay, the
yellow colour being due to the iron content in Biotite. The


