3429
Technical Committee 307 + 212 /
Comité technique 307 + 212
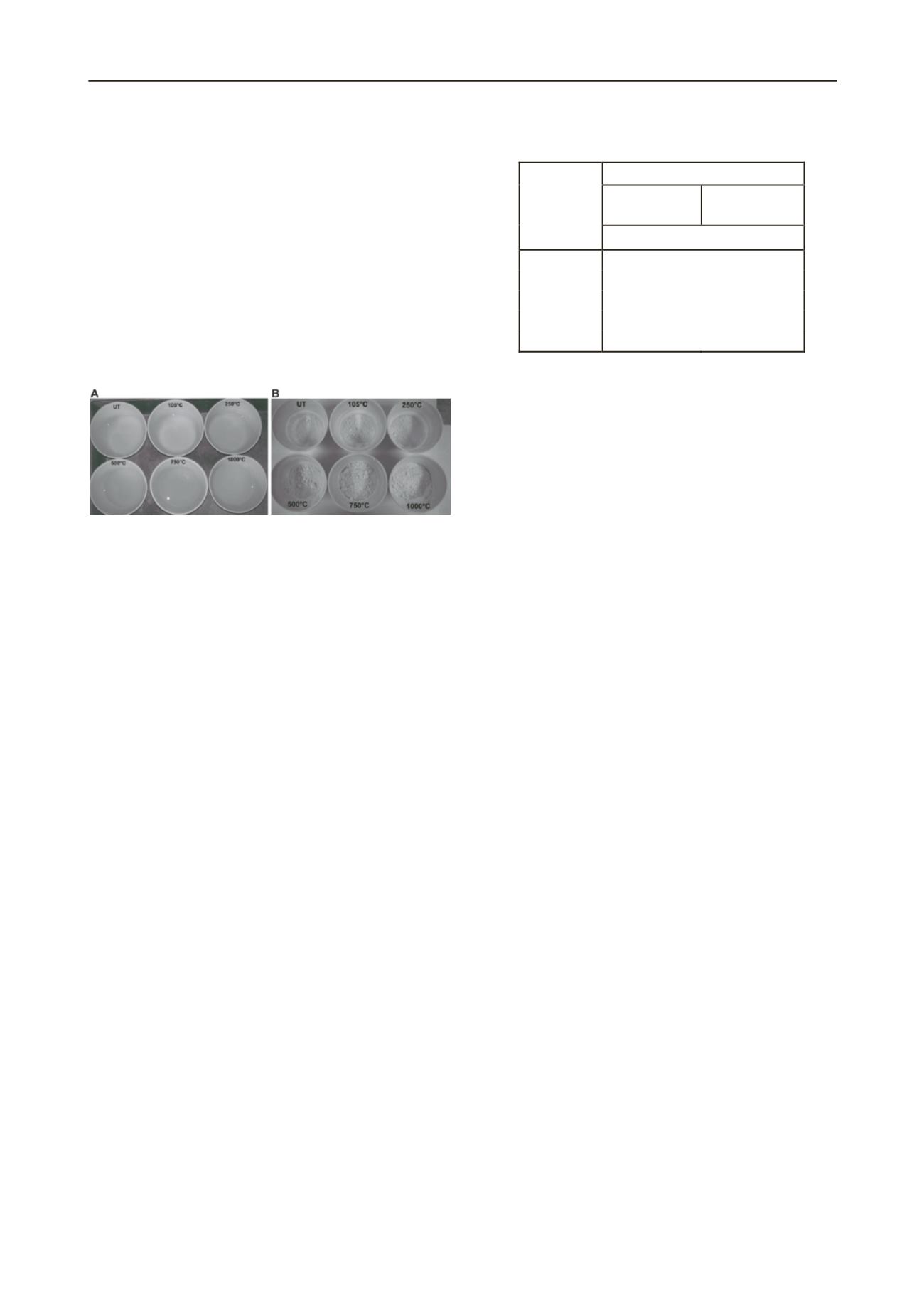
During testing that required the addition of distilled water, the
clay was observed to discolour in the mixed samples, but the
distilled water stayed clear (Figure 2). This is very likely
associated with the iron oxidation reaction described above. It is
possible that this surface reaction enables some of the iron
oxides to become mobile and attach themselves onto the clay
particles causing this discolouration (Zhang et al., 2011). In the
clay-only samples, slight colour changes from white to greyish
white were observed
.
In the smouldered samples for 10% clay
and 20% clay mixtures with sand, the colour change was to a
darker grey than the heat-only samples. This colour change was
likely influenced by staining from the coal tar as well as the
inherent colour change of the kaolin.
Figure 2. A: Kaolin clay (sand-clay mixture) fraction after heat
treatment; B: Kaolin only after heat treatment.
3.2. Particle Size Distribution and Densities
In contrast to mineralogy, elevated temperatures did not seem to
affect the particle density or minimum/maximum bulk densities
of the silica sand. No real relationship was apparent between
treatment temperature and density. For the particle density, the
values are consistently near 2.65mg/m
3
, which is a value that is
widely used in geotechnical engineering calculations. The
maximum and minimum densities are equally unaffected by
heat treatment or smouldering. These observations are not
consistent with the literature on wild and forest fire effects on
soil properties, which suggests that bulk density would increase
with temperature (Are et al., 2009; Certini, 2005). The lack of
organic matter may explain the contrast. The results in this
study, which show no significant change in density, suggest that
the changes in soil density that are observed after wildfires are
associated primarily with effects on organic matter and
potentially the smaller silt and clay–sized particles.
Heat treatment has a small but appreciable effect on particle
size distribution. As exposure temperature increases from 250 to
1000°C, the sample retained on the 1.18mm sieve increases.
The variation in particle size distribution may be linked to the
loss of mass beyond the initial moisture content. As temperature
increases, mass loss increases. Although there is a dehydration
reaction from goethite to hematite in the sand, the fraction of
iron oxide relative to the total composition of the sand is too
small for this reaction alone to account for the whole additional
mass loss. For the silica sand kaolin clay mixture the trend is
slightly different (Table 2). The sample retained on the 1.18mm
sieve increases very slightly for 250°C, followed by an overall
decrease for 250, 500, 750 and 1000°C. For 105 and 250°C the
clay coats the sand grains allowing them to be retained on the
1.18mm sieve, for temperatures above 500°C this coating is
destroyed resulting in less sample being retained. The coating
effect increases the sand fraction >1.18mm by 7 to 16%
compared to the higher temperature samples. This is not an
increase in the sand fraction but an increase in grains the size of
this fraction due to the additional clay coating. This coating
could have an impact on the permeability and shear behaviour
of these lower temperature samples after heat treatment
depending on how easily it can be destroyed or removed by
grain interaction or interaction with water.
Table 2. Sieve analysis results for silica sand – 10% kaolin clay
mixtures (5% MC) for different heat treatments
SIEVE ANALYSIS
1.18mm <1.18mm
Sample
% retained
105
81.8
±
1.9
18.2
±
2.1
250
82.7
±
0.8
17.3
±
1.0
500
74.5
±
3.2
25.5
±
3.6
750
65.6
±
3.6
34.4
±
3.7
1000
67.7
±
0.8
32.3
±
1.5
3.3. Atterberg Limits for kaolin clay
High temperature processes impact the dynamic properties of
soils, particularly liquid and plastic limits at the highest
temperatures. This impact on the clay fraction can lead to
changes in dynamic behaviour for the clay – sand mixtures. The
Atterberg limits for the temperature treatment up to 500°C are
similar, especially the liquid limits are all within 64±2%, where
the liquid limit for 750°C increases to 81% (Table 3) and this
clay has a very high plasticity range compared to the lower
temperatures. This is likely due to the increased dehydration of
the clay at this temperature. These results are in contrast to Tan
et al (2004) (Tan et al., 2004) who recorded an decrease in both
liquid and plastic limits with increasing temperature treatment,
including non-plastic behaviour for the clays above 400°C. This
difference in behaviour can be two-fold. Firstly it can an affect
based on the state of the tested sample, especially in regards to
initial moisture content. Tan et al (5) uses over consolidated
natural clays from Turkey, where this study investigated
commercial loose kaolin powder with no moisture content.
Secondly, the behaviour can be based on the main mineral
contained in the sample, montmorillonite (2:1 clay) for the
natural clays from Turkey compared to kaolinite (1:1 clay) for
the commercial powder samples. Kaolininte does not swell in
the presence of water whereas montmorillonite does swell.
Based on this distinction, the responses of montmorillonite and
other swelling clays to heat treatment may be different from the
responses of kaolinite. Further work is necessary to explore the
responses of montmorillonite and other clay minerals during
thermal and smouldering remediation processes. The liquid
limit test for the sample treated at 1000°C was not possible due
to the clay not mixing properly with the water and behaving
slightly non-newtonian, which means as the mixing motion
stopped the sample liquefied and it was impossible to create a
testable sample. Initially, the clay mixed well with the water and
it was possible to produce a paste but with increasing water
content the behaviour changed and the sample only stayed solid
under a constant mixing motion, after stopping the mixing the
sample quickly liquefied and dispersed. Storage in a sealed
container did not yield different results. In contrast to the other
samples (105-750°C treatments), no clay paste was formed.
Instead, a stiff clay layer formed at the bottom of the bag with
an overlying layer of clean water (Figure 3). This is an
unexpected behaviour of the clay and no explanation has been
found in the literature. It is likely that the temperature of
1000°C causes de-hydroxylation of the clay minerals, followed
by aggregation of the particles and sintering (Fabbri et al.). The
net result is that the kaolin particles seem to become
hydrophobic. The induced hydrophobicity will affect dynamic
properties of the soil such as grain-grain and grain-water
interactions. In swelling clays, the effects are expected to be
similar to those observed in kaolinite, though based on previous
work (Tan et al., 2004), the shift toward hydrophobic particles
may occur at lower temperatures. Because other clays are
swelling, the effects of the dehydration and melting reactions


