3428
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
changes from yellowish brown to reddish brown. This is due to
the oxidation of soil iron content from goethite to maghemite or
hematite (Goforth et al., 2005; Ketterings and Bigham, 2000).
Decomposition of soil particles, especially clay minerals, starts
at temperatures above 550°C (Certini, 2005). These
temperatures are rarely reported for wild and forest fire, but
temperatures up to 1200°C can be achieved during smouldering
remediation (Pironi et al., 2009; Switzer et al., 2009).
This study aims to characterise the effects of moderate and
high temperatures as well as smouldering on soil properties to
determine the impact changes will have to the soil and predict
possible complications that may arise during or after
remediation treatment. Silica sand and kaolin clay are used as
constituents of a synthesised simple soil. Clean untreated, heat-
treated and contaminated/smouldered materials are evaluated to
determine the impacts of the treatment conditions on soil
properties.
2. MATERIALS AND METHODS
Coarse silica sand (Leighton Buzzard 8/16, Sibelco, Sandbach,
UK) and kaolin clay (Whitchem Ltd, UK) were used as the base
soil for all of the experiments. The sand contains 99% silicon-
dioxide, has a mean grain size of 1.34 and a bulk density of
1.7g/cm
3
(Switzer et al., 2009). The sand and clay were
accepted as received and the sand was subjected to the same
pre-treatment. A programmable muffle furnace (Nabertherm
L9/11/SKM, Nabertherm GmbH, Lilienthal, Germany) was
used for all heating experiments. The sands evaluated after
smouldering remediation were prepared in a 3m
3
experiment
involving coal tar mixed with coarse sand. The initial
concentration of this mixture was 31000 ± 14000 mg/kg total
extractable petroleum hydrocarbons before treatment and the
average concentration after smouldering remediation across the
majority of the vessel was 10 ± 4 mg/kg (Pironi et al., 2009).
Table 1. Heat treatment programs
2.1 Sample Preparation and Heat Treatment
The silica sand was washed and wet sieved using a 425µm
screen to eliminate any loose fines and air dried for several
days. In case of mixed samples the dried silica sand was mixed
with 10% mass kaolin clay and 5% moisture content before
being heat treated. For each test, the required amount of samples
was heated in the furnace following the heat treatment
programmes listed in Table 1. After the required exposure
duration, the samples were removed from the muffle furnace
and placed in a desiccator to cool. Samples heated to
temperatures above 500°C were allowed to cool in the furnace
to 200°C before transfer to the desiccator.
2.2 Laboratory Testing
Particle density was measured using the gas-jar method suitable
for coarse soils. Minimum density was measured using 1000g
of sand in a 1L glass measuring cylinder with 20mL graduation
BS1377-2:1990 and BS1377-4:1990). Maximum density was
determined using the vibrating hammer method (BS1377-
4:199). Particle size distribution for the sand was determined
using a sieving method (BS1377-2:1990) using 1.18mm,
600µm, 425µm, 300µm and 212µm sieve sizes. The Atterberg
Limits for the clay were determined using the cone penetration
and rolling methods as outlined in BS1377-2:1990.
The sand-clay mixtures were prepared by dry-mixing 90%
sand and 10% clay (by mass) and then adding distilled water to
achieve a 5% moisture content. The sample was then thoroughly
kneaded in a plastic bag by hand for 10 minutes and allowed to
rest for 2 hours before any heat treatment.
3. RESULTS AND DISCUSSION
3.1. Mineralogy
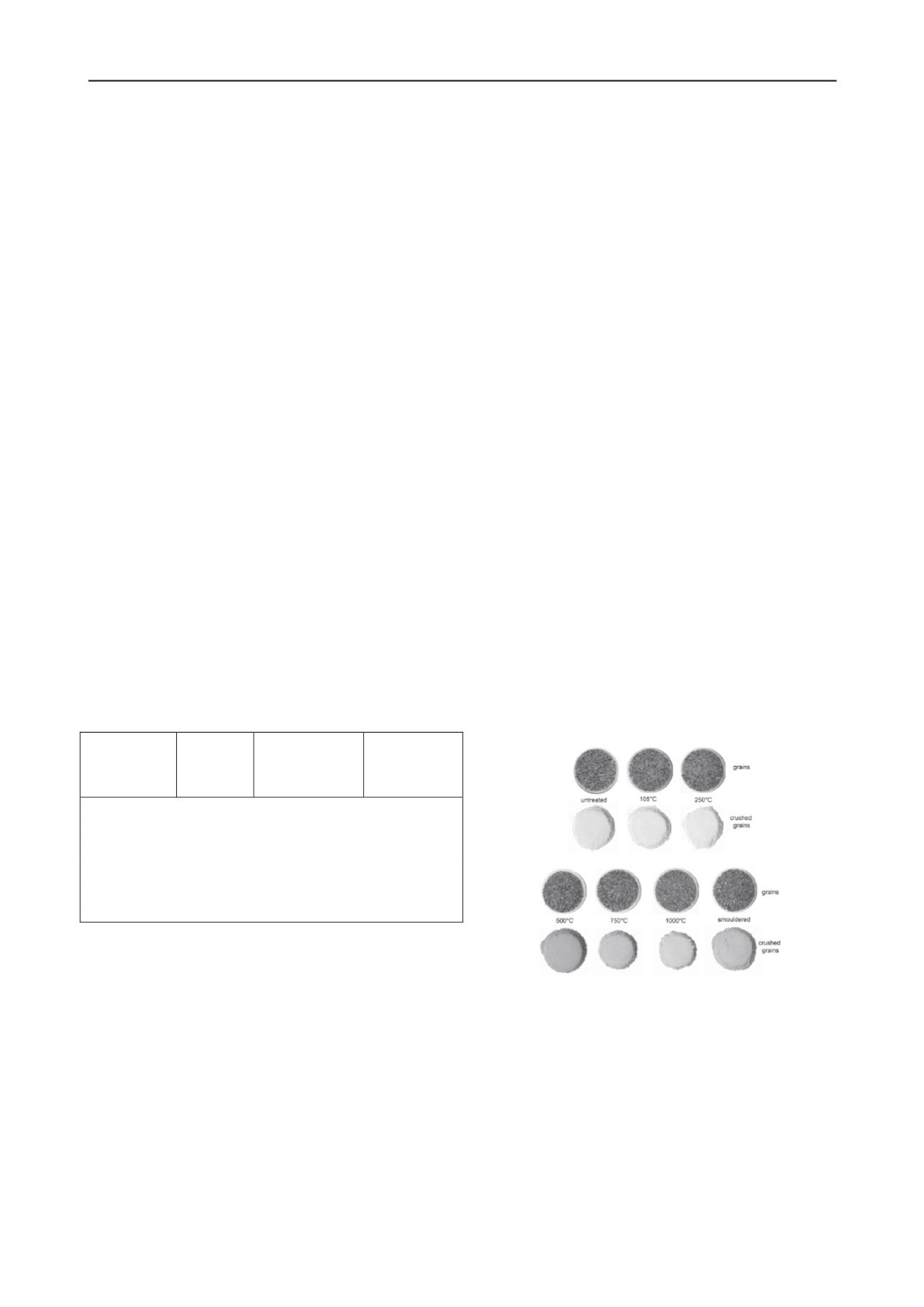
During the heat treatment testing and after smouldering
remediation, a colour change of the silica sand was observed
(Figure 1). Exposure of this material to high temperatures
results in colour change from yellowish brown to reddish brown
with increasing temperature for the silica sand grains and a
change from yellow to pinkish red for the crushed silica sand.
This colour change is associated with the dehydration reaction
of goethite with increasing temperatures to form hematite or
maghemite. During the dehydration, the density of the iron-
hydroxide increases from 4.3 mg/m
3
for goethite to 5.2 mg/m
3
for hematite (Wenk and Bulakh, 2004). The sand is comprised
primarily of silicon dioxide; iron oxides make up a small
fraction of its composition. High temperatures may cause
additional changes in mineralogy that may be less likely to be
detected by visual examination (Goforth et al., 2005; Pomiès et
al., 1998). For example, silicon dioxide becomes unstable with
high temperatures and forms silica polymorphs such as
trydimite or cristobalite (Hand et al., 1998; Wenk and Bulakh,
2004). Thermal treatments (100-1200°C) on fly ash have
transformed quartz minerals to cristobalite and smaller particles
exhibit a characteristic glassy composition due their faster
cooling time (Mollah et al., 1999).
Figure 1. Silica Sand grains and crushed grains after heat treatment.
Sample
Name
Pre-
heating
time
(min)
Peak
temperature
for 60min
cooling
down time
(min)
Untreated
105
30
105°C (24h)
0
250
30
250°C
0
500
30
500°C
~ 60
750
60
750°C
~ 180
1000
60
1000°C
~ 240


