3078
Proceedings of the 18
th
International Conference on Soil Mechanics and Geotechnical Engineering, Paris 2013
steps. The first step is denaturation, which involves the highest
temperature in the cycle (typically 94-95
C; Promega, 2012;
Roche, 2011a). This separates the strands of double stranded
DNA to act a template for DNA synthesis. The second step is
annealing, which involves the lowest temperature in the cycle.
In this step PCR primers become attached to the template DNA.
PCR primers are short fragments of DNA which are designed
and synthesised to match to the ends of a target section of DNA,
and serve as a starting point for DNA replication. The annealing
temperature depends on the properties of the primers being
used, but is usually 42–65
C (Brown, 2001). The third step is
extension where double stranded DNA is reconstructed base-
pair by base-pair from dNTPs in the reaction mixture by the
polymerase enzyme acting at the 3’ end of the annealed primer
(typical temperature 68-72
C). This three step cycle is repeated
many times, with the amount of the target DNA fragment
doubling (in theory) in each cycle. In practice there is initially
exponential amplification, but it levels off with increasing
numbers of cycles as the polymerase enzyme loses activity and
the reagents (dNTPs and primers) are consumed until,
eventually, no further product is produced. If the primers have
been appropriately designed and the reaction conditions
optimised only the target DNA fragment should be amplified.
The very high amplifications achieved by repeated cycling
make PCR a very powerful technique, but users need to be
aware of potential artefacts that can arise. The three main ones
are contamination, polymerase errors and bias. The highly
sensitive nature of PCR means that even low levels of
contaminating DNA (from other samples or the laboratory
environment) can lead to amplification of products that don’t
originate from the sample. Thus scrupulous cleanliness and the
use of negative controls (where no DNA sample is added to the
reaction) are mandatory. Small errors and slight biases in a
single amplification cycle can over the course of many cycles
lead to gross distortions in the representation of different
fragments in the final PCR product. Thus, as a rule, data from
an analysis involving a PCR reaction should be treated as
qualitative rather than quantitative (the exception being where
the more advanced tool of qPCR is used). PCR errors can arise
due DNA polymerase errors (the Taq polymerase error rate is
~3x10
-5
per nucleotide per duplication; Acinas et al. 2004), the
formation of chimeric molecules, and the formation of
heteroduplex molecules. The best way to avoid this type of
problem is to avoid unnecessary over-amplification because
such errors are cumulative (i.e. use the smallest possible number
of PCR amplification cycles compatible with the intended
application; Qiu et al. 2001; Acinas et al. 2005). For some
purposes it may also be necessary to use a “proof-reading”
polymerase enzyme with a far lower intrinsic error rate than
Taq. PCR bias arises because of intrinsic differences in the
amplification efficiency of different templates (e.g. due to
differences in the GC-content). In late stages of amplification
self-annealing of the most abundant templates can hinder their
further amplification. PCR bias is reduced by using high
template concentrations, performing fewer PCR cycles (Polz &
Cavanaugh 1998) and by using a thermocycler that ramps
quickly between the cycling temperatures (Acinas et al. 2005).
In Geo-Environmental Engineering PCR is most frequently
used to characterise microorganisms or microbial populations.
PCR is often used to amplify the same section of the same gene
of different species of microorganisms in an environmental
sample. The 16S rRNA gene is frequently used to identify
microbes, and to study diversity, because it is present in all
prokaryotic organisms (those without a nucleus, like bacteria).
In this case the primers are targeted at two conserved regions of
the gene where the base sequence is almost identical between
widely different organisms because that part of the molecule
encodes a function necessary for life so that the intervening
region, which is more divergent, is amplified. The difference in
sequence between the divergent regions can be used to infer
evolutionary distance. PCR is also used to amplify sections of
DNA between genes since these are often very variable in
length and/or base sequence even for quite closely related
species. An example of this approach is rRNA Intergenic Spacer
analysis (RISA), which is often used for community
fingerprinting with the aim of identifying when there has been a
significant change in the microbial population.
3 CHARACTERISING MICROBIAL POPULATIONS
Simple PCR based techniques can identify that a particular gene
is present within a microbial population, and to “fingerprint”
bacterial populations to identify significant changes in popula-
tion over time or under different conditions (section 4), but
actually identifying the bacterial species present in a sample
requires some method of separating out the individual DNA
fragments in an environmental sample, and sequencing them.
The traditional approach to this problem is “cloning and
sequencing” (see e.g. Islam et al. 2004). This approach starts
with a PCR reaction on environmental DNA using broad
specificity primers that target a suitable gene (usually the 16S
rRNA gene). The resulting PCR product contains multiple
copies of the target gene from all the species in the sample.
These double stranded DNA fragments are then ligated to
(joined-into) a standard cloning vector (e.g. pGEM-T, TOPO,
etc.) to form circular double-stranded DNA molecules called
plasmids. This is achieved with standard molecular biology kits
available from suppliers such as Promega or Life Technologies.
Each plasmid will contain a different DNA fragment from the
PCR reaction. The plasmid is then “transformed” (inserted) into
specially weakened laboratory strains of E-coli that lack
resistance to antibiotics. The standard cloning vectors are
designed to confer antibiotic resistance to any cell into which
they are inserted, allowing selection of those cells that take up a
plasmid. An important feature of the transformation is that it is
very inefficient (which is why selection is necessary), and thus
it is unlikely that more than one plasmid is inserted into a cell.
The cells are then plated out on agar plates containing the
antibiotic, so that only cells containing the plasmid grow. If this
is done with care then bacterial colonies will grow on the plates
so that each colony has grown from a single cell, and will thus
contain copies of a single DNA fragment from the
environmental sample. These cells can be harvested, and the
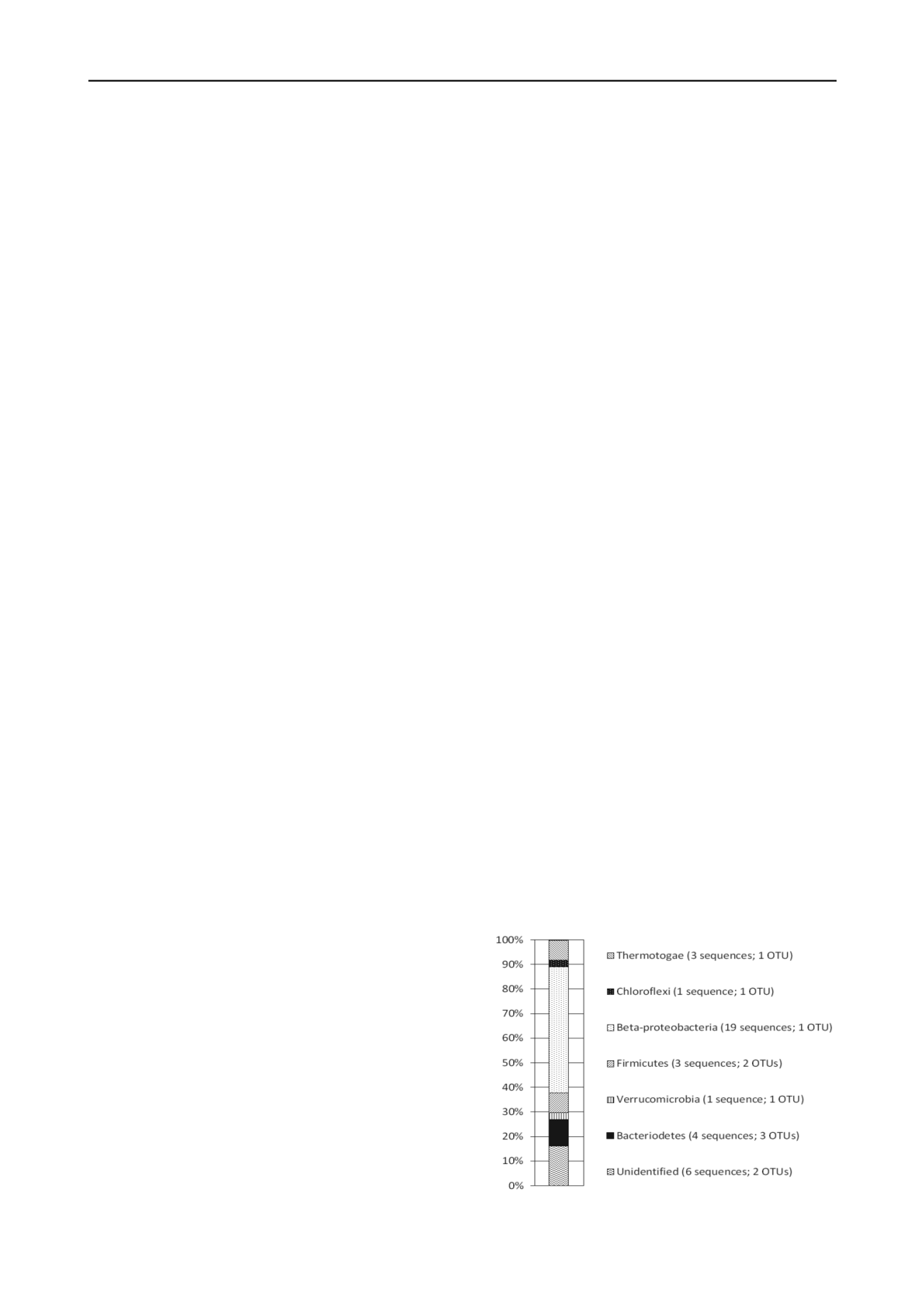
plasmid they contain sent for gene sequencing. Figure 1 shows
the bacterial population of soil from near a lime kiln waste tip
determined by this technique (Burke et al. 2012). This showed
that the bacterial population of the sample of buried soil was
dominated by a single, unidentified species within the
Comamonadaceae family of β-proteobacteria. Determination of
the geochemical conditions allowed this study to postulate a
link between anaerobic respiration of this specie and the
Figure 1. Phylogenetic diversity of 16S rRNA gene sequences extracted
from sample. Key shows the number of OTUs within each phylum


